Pfizer 2007 Annual Report Download - page 4
Download and view the complete annual report
Please find page 4 of the 2007 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
2 2007 Financial Report
Financial Review
Pfizer Inc and Subsidiary Companies
medicines that led their therapeutic areas based on revenues.
(See further discussion in the “Analysis of the Consolidated
Statement of Income” section of this Financial Review.)
•Decision to Exit Exubera:
Exubera was the first inhaled insulin therapy for the treatment
of diabetes, and since May 2006, had been launched in
Germany, Ireland, the U.K. and the U.S. In the third quarter of
2007, after an assessment of the financial performance of
Exubera, as well as its lack of acceptance by patients, physicians
and payers, we decided to exit the product.
Our Exubera-related exit plans included working with physicians
over a three-month period to transition patients to other
treatment options, evaluating redeployment options for
colleagues, working with our partners and vendors with respect
to transition and exit activities, working with regulators on
concluding outstanding clinical trials, implementing an
extended transition program for those patients unable to
transition to other medications within the three-month period,
and exploring asset disposal or redeployment opportunities, as
appropriate, among other activities.
As part of this exit plan, in 2007, we paid $135 million to one
of our partners in satisfaction of all remaining obligations
under existing agreements relating to Exubera and a next
generation insulin (NGI) under development. In addition, in the
event that a new partner is selected, we have agreed to transfer
our remaining rights and all economic benefits for Exubera and
NGI. This transfer of our interests would include the transfer of
the Exubera New Drug Application and Investigational New
Drug Applications and all non-U.S. regulatory filings and
applications, continuation of ongoing Exubera clinical trials and
certain supply chain transition activities.
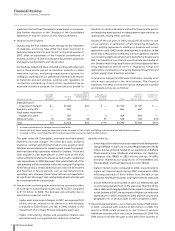
Total pre-tax charges for 2007 were $2.8 billion, virtually all of
which were recorded in the third quarter. The financial
statement line items in which the various charges are recorded
and related activity are as follows:
SELLING
CUSTOMER INFORMATIONAL & RESEARCH & ACTIVITY ACCRUAL AS
RETURNS- COST OF ADMINISTRATIVE DEVELOPMENT THROUGH OF DEC. 31,
(MILLIONS OF DOLLARS) REVENUES SALES EXPENSES EXPENSES TOTAL DEC. 31, 2007(a) 2007
Intangible asset
impairment charges(b) $— $1,064 $41 $ — $1,105 $1,105 $ —
Inventory write-offs — 661 — — 661 661 —
Fixed assets impairment
charges and other — 451 — 3 454 454 —
Other exit costs 10 427 44 97 578 164 414(c)
Total $10 $2,603 $85 $100 $2,798 $2,384 $414
(a) Includes adjustments for foreign currency translation.
(b) Amortization of these assets had previously been recorded in
Cost of sales
and
Selling, informational and administrative expenses
.
(c) Included in
Other current liabilities
($375 million) and
Other noncurrent liabilities
($39 million).
The asset write-offs (intangibles, inventory and fixed assets)
represent non-cash charges. The other exit costs, primarily
severance, contract and other termination costs, as well as other
liabilities, are associated with marketing and research programs,
and manufacturing operations related to Exubera. These exit
costs resulted in cash expenditures in 2007 (such as the $135
million settlement referred to above) and will result in additional
cash expenditures in 2008. We expect that substantially all of the
cash spending will be completed within the next year. During the
exit of this product, certain additional cash costs will be incurred
and reported in future periods, such as maintenance-level
operating costs. However, those future costs are not expected to
be significant. We expect that substantially all exit activities will
be completed within the next year.
•Income from continuing operations before cumulative effect
of a change in accounting principles was $8.2 billion compared
to $11.0 billion in 2006. The decrease was primarily due to
event-driven expenses, such as:
䡬higher asset impairment charges. In 2007, we expensed $2.8
billion, pre-tax, related to our decision to exit Exubera,
compared to $320 million, pre-tax, in 2006, related to the
impairment of our Depo-Provera intangible asset; and
䡬higher restructuring charges and acquisition-related costs
associated with our expanded cost-reduction initiatives,
partially offset by:
䡬lower Acquisition-related in-process research and development
charges (IPR&D). In 2007, we incurred IPR&D expenses of $283
million, pre-tax, primarily related to our acquisitions of BioRexis
Pharmaceutical Corp. (BioRexis) and Embrex, Inc. (Embrex),
compared with IPR&D of $835 million, pre-tax, in 2006,
primarily related to our acquisitions of PowderMed Ltd.
(PowderMed), and Rinat Neuroscience Corp. (Rinat);
䡬higher interest income compared to 2006, due primarily to
higher net financial assets during 2007 compared to 2006,
reflecting proceeds of $16.6 billion from the sale of our
Consumer Healthcare business, and higher interest rates; and
䡬a lower effective income tax rate. In 2007, our effective tax rate
on continuing operations of 11.0% was lower than the 15.3%
rate in 2006, which largely reflects the tax impact of our decision
to exit Exubera in 2007, the tax impact of higher cost-reduction
expenditures in 2007 compared to 2006 and the volume and
geographic mix of product sales in 2007 compared to 2006.
•Discontinued operations—net of tax were losses of $69 million
in 2007, compared with income of $8.3 billion in 2006. The
results in 2006 relate primarily to our former Consumer
Healthcare business, which was sold on December 20, 2006. The
2006 amount includes the gain on the sale of this business of