Danaher 2009 Annual Report Download - page 16
Download and view the complete annual report
Please find page 16 of the 2009 Danaher annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Table of Contents
Medical Devices
Certain of our products are medical devices that are subject to regulation by the United States Food and Drug Administration (the “FDA”) and by the
comparable agencies of the non-U.S. countries where our products are sold. Some of the regulatory requirements of these foreign countries are different than
those applicable in the United States.
Pursuant to the Federal Food, Drug, and Cosmetic Act (the “FDCA”), the FDA regulates virtually all phases of the development, manufacture, sale and
distribution of medical devices, including their introduction into interstate commerce, manufacture, advertising, labeling, packaging, marketing, distribution
and record keeping. Pursuant to the FDCA and FDA regulations, certain facilities of our operating subsidiaries are registered with the FDA as medical device
manufacturing establishments. The FDA, as well as industrial standards bodies such as the International Standards Organization (“ISO”), regularly inspect
our registered and/or certified facilities.
We sell both Class I and Class II medical devices. A medical device, whether exempt from, or cleared pursuant to, the premarket notification requirements of
the FDCA, or approved pursuant to a premarket approval application, is subject to ongoing regulatory oversight by the FDA to ensure compliance with
regulatory requirements, including, but not limited to, product labeling requirements and limitations, including those related to promotion and marketing
efforts, quality system requirements and medical device (adverse event) reporting. Certain of our products utilize radioactive material, and we are subject to
federal, state and local regulations governing the management, storage, handling and disposal of these materials. In addition, we are subject to various federal,
state and local laws targeting fraud and abuse in the healthcare industry, including anti-kickback and false claims laws. For a discussion of risks related to
our regulation by the FDA and comparable agencies of other countries, and other regulatory regimes referenced above, please refer to “Item 1A. Risk Factors.”
Export/Import Compliance
We are required to comply with various U.S. export/import control and economic sanctions laws, including:
• the International Traffic in Arms Regulations administered by the U.S. Department of State, Directorate of Defense Trade Controls, which, among
other things, imposes license requirements on the export from the United States of defense articles and defense services (which are items
specifically designed or adapted for a military application and/or listed on the United States Munitions List);
• the Export Administration Regulations administered by the U.S. Department of Commerce, Bureau of Industry and Security, which, among other
things, impose licensing requirements on the export or re-export of certain dual-use goods, technology and software (which are items that
potentially have both commercial and military applications);
• the regulations administered by the U.S. Department of Treasury, Office of Foreign Assets Control, which implement economic sanctions
imposed against designated countries, governments and persons based on United States foreign policy and national security considerations; and
• the import regulatory activities of the U.S. Customs and Border Protection.
Other nation’s governments have also implemented similar export and import control regulations, which may affect our operations or transactions subject to
their jurisdictions. For a discussion of risks related to export/import control and economic sanctions laws, please refer to “Item 1A. Risk Factors.”
International Operations
Our products and services are available worldwide, and our principal markets outside the United States are in Europe and Asia. We believe this geographic
diversity allows us to draw on the skills of a worldwide workforce, provides stability to our operations, allows us to drive economies of scale, provides
revenue streams that may help offset economic trends that are specific to individual economies and offers us an opportunity to access new markets for
products. In addition, we believe that our future growth depends in part on our ability to develop products and sales models that target developing countries.
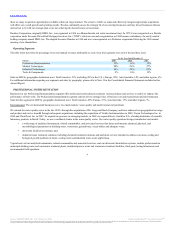
The table below describes annual revenue derived from customers outside the U.S. as a percentage of total annual revenue for each of the last three years, by
segment and in the aggregate:
Professional Instrumentation 54% 57% 55%
Medical Technologies 65% 64% 63%
Industrial Technologies 50% 51% 50%
Tools & Components 16% 19% 17%
Total percentage of revenue derived from customers outside of the United States 52% 53% 51%
14
Source: DANAHER CORP /DE/, 10-K, February 24, 2010 Powered by Morningstar® Document Research℠
The information contained herein may not be copied, adapted or distributed and is not warranted to be accurate, complete or timely. The user assumes all risks for any damages or losses arising from any use of this information,
except to the extent such damages or losses cannot be limited or excluded by applicable law. Past financial performance is no guarantee of future results.