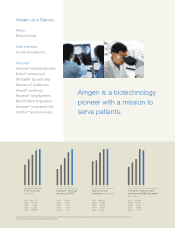
Amgen 2007 Annual Report Download - page 8
Download and view the complete annual report
Please find page 8 of the 2007 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
6
(a) To exclude, for the applicable periods, the non-cash expense associated with
writing-off the acquired in-process R&D related to the acquisitions of Alantos
Pharmaceutical Holding, Inc. (“Alantos”) and Ilypsa, Inc. (“Ilypsa”) in 2007,
Abgenix, Inc. (“Abgenix”) and Avidia, Inc. (“Avidia”) in 2006, Tularik Inc. (“Tularik”)
in 2004, and Immunex Corporation (“Immunex”) in 2002.
(b) To exclude restructuring related costs primarily including, as applicable, asset
impairment charges, staff separation costs, accelerated depreciation and loss
accruals for certain leases.
(c) To exclude the ongoing, non-cash amortization of acquired product technology
rights, primarily ENBREL, related to the Immunex acquisition.
(d) To exclude the impact of stock option expense recorded in accordance with
Statement of Financial Accounting Standards (“SFAS”) No. 123R. Effective
January 1, 2006, Amgen adopted SFAS No. 123R.
(e) To exclude the income tax benefit recognized as the result of resolving certain
non-routine transfer pricing issues with the Internal Revenue Service for prior periods.
( f ) To exclude the write-off of inventory principally due to changing regulatory and
reimbursement environments.
(g) To exclude, for the applicable periods, the ongoing, non-cash amortization of the
R&D technology intangible assets acquired with the acquisitions of Abgenix and Avidia.
(h) To exclude the pro rata portion of the deferred financing and related costs that
were immediately charged to interest expense as a result of certain holders of
the convertible notes due in 2032 exercising their March 1, 2007 put option and
the related convertible notes being repaid in cash.
( i ) To exclude merger related expenses incurred, due to the Alantos, Ilypsa, Abgenix,
Avidia, Tularik and Immunex acquisitions, primarily related to incremental costs
associated with retention, integration and/or recording inventory acquired at fair value
which is in excess of our manufacturing cost for the applicable acquisitions and periods.
( j ) To exclude, for the applicable periods, loss accruals, awards, or cost recoveries for
legal settlements.
(k) To exclude the impact of writing-off the cost of a semi-completed manufacturing asset
that will not be used due to a change in manufacturing strategy.
( l ) To exclude severance related expenses incurred in connection with our acquisition of
the remaining 51 percent ownership interest of Dompé Biotec, S.p.A.
(m) To exclude the impairment of a non-ENBREL related intangible asset previously
acquired in the Immunex acquisition.
(n ) Pursuant to the if-converted method of calculating EPS, the numerator for “Adjusted”
EPS in 2002 reflects the avoidance of interest expense incurred, net of tax, related to
the assumed conversion of the convertible notes. The conversion of such debt and the
avoidance of interest expense is not assumed for calculating the GAAP EPS because its
impact is anti-dilutive due to the GAAP net loss in 2002.
(o) Due to the GAAP net loss in 2002, shares used in calculating the GAAP loss per share
exclude the impact of stock options and convertible notes because their impact was
anti-dilutive. Shares used in calculating the “Adjusted” earnings per share for 2002
include the impact of dilutive stock options (27 million shares) and convertible notes
(29 million shares) under the treasury stock and if-converted methods, respectively.
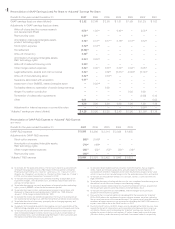
Results for the years ended December 31, 2007 2006 2005 2004 2003 2002 2001
GAAP earnings (loss) per share (diluted) $ 2.82 $ 2.48 $ 2.93 $ 1.81 $ 1.69 $ (1.21)$ 1.03
Adjustments to GAAP earnings (loss) per share:
Write-off of acquired in-process research
and development (R&D) 0.53(a) 1.03(a) — 0.42(a) —2.53 (a) —
Restructuring costs 0.51(b) ———— ——
Amortization of acquired intangible assets,
product technology rights 0.16(c) 0.17(c) 0.17(c) 0.16(c) 0.17(c) 0.12(c) —
Stock option expense 0.12(d) 0.14(d) ——— ——
Tax settlement (0.08)(e) ———— ——
Write-off of inventory 0.08(f) ———— ——
Amortization of acquired intangible assets,
R&D technology rights 0.04(g) 0.03(g) ——— ——
Write-off of deferred financing costs 0.03(h) ———— ——
Other merger-related expenses 0.02(i) 0.02(i) 0.01(i) 0.02(i) 0.04(i) 0.06 (i) —
Legal settlements, awards and cost recoveries 0.02(j) — 0.02(j) (0.01)(j) (0.02)(j) (0.12 )(j) —
Write-off of manufacturing asset 0.02(k) — 0.04(k) — — ——
Severance associated with acquisition 0.01(l) ———— ——
Impairment of non-ENBREL related intangible asset —0.03(m) ——— ——
Tax liability related to repatriation of certain foreign earnings —— 0.03 — — ——
Amgen Foundation contribution —— — — 0.02 0.03 —
Termination of collaboration agreements —————(0.03) 0.12
Other 0.01 — — — — —0.03
4.29 3.90 3.20 2.40 1.90 1.38 1.18
Adjustment for interest expense on convertible notes —————0.01(n) —
“Adjusted” earnings per share (diluted) $ 4.29 $ 3.90 $ 3.20 $ 2.40 $ 1.90 $ 1.39(o) $ 1.18
Reconciliation of GAAP Earnings (Loss) Per Share to “Adjusted” Earnings Per Share
Results for the years ended December 31, 2007 2006 2005 2004 2003
GAAP R&D expense $ 3,266 $ 3,366 $ 2,314 $ 2,028 $ 1,655
Adjustments to GAAP R&D expense:
Stock option expense (83)(d) (104)(d ) ———
Amortization of acquired intangible assets,
R&D technology rights (71)(g) (48)(g) ———
Other merger-related expenses (29)(i) (23)(i) (12)(i) (32)(i) (34)(i)
Restructuring costs (19)(b) ————
“Adjusted” R&D expense $ 3,064 $ 3,191 $ 2,302 $ 1,996 $ 1,621
Reconciliation of GAAP R&D Expense to “Adjusted” R&D Expense
($ in millions)