Amgen 2007 Annual Report Download - page 13
Download and view the complete annual report
Please find page 13 of the 2007 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.PART I
Item 1. BUSINESS
Overview
Amgen Inc. (including its subsidiaries, referred to as “Amgen,” “the Company,” “we,” “our” and “us”) was
incorporated in 1980 and is a global biotechnology company that discovers, develops, manufactures and markets
human therapeutics based on advances in cellular and molecular biology. We operate in one business segment —
human therapeutics.
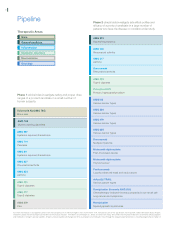
We market human therapeutic products in the areas of supportive cancer care, nephrology, inflammation
and oncology. Our principal products include Aranesp®(darbepoetin alfa), EPOGEN®(Epoetin alfa), Neulasta®
(pegfilgrastim), NEUPOGEN®(Filgrastim) and Enbrel®(etanercept). Aranesp®and EPOGEN®stimulate the
production of red blood cells to treat anemia and belong to a class of drugs referred to as erythropoiesis-
stimulating agents (“ESAs”). Aranesp®is used for the treatment of anemia both in supportive cancer care and in
nephrology. EPOGEN®is used to treat anemia associated with chronic renal failure (“CRF”). Neulasta®and
NEUPOGEN®selectively stimulate the production of neutrophils, one type of white blood cell that helps the
body fight infections. ENBREL blocks the biologic activity of tumor necrosis factor (“TNF”) by inhibiting TNF,
a substance induced in response to inflammatory and immunological responses, such as rheumatoid arthritis and
psoriasis. For the years ended December 31, 2007, 2006 and 2005, our principal products represented 95%, 97%
and 98% of total product sales, respectively.
We operate in a highly regulated industry and various U.S. and foreign regulatory bodies have substantial
authority over how we conduct our business in those countries. Government authorities in the United States and
in other countries regulate the manufacturing and marketing of our products and our ongoing research and devel-
opment (“R&D”) activities. (See “Government Regulations.”) For example, prior to obtaining regulatory
approval to market a product, we must conduct extensive clinical studies designed to establish the safety and ef-
fectiveness of the product candidate for use in humans in the indications sought. Furthermore, in order to
maintain regulatory approval to market a product, we may be required to conduct further clinical trials and to
provide additional information on safety and effectiveness. (See “Postmarketing and Safety Activities.”) The
regulatory environment is evolving and there is increased scrutiny on drug safety and increased authority being
granted to regulatory bodies, in particular the U.S. Food and Drug Administration (“FDA”), to assist in ensuring
the safety of therapeutic products.
Most patients receiving our principal products for approved indications are covered by either government
and/or private payer health care programs. The reimbursement environment is evolving with greater emphasis on
cost containment. Therefore, sales of our products are and will continue to be affected by the availability and ex-
tent of reimbursement from third-party payers, including government and private insurance plans and
administration of those programs. Governments may regulate access to, prices or reimbursement levels of our
products to control costs or to affect levels of use of our products. Worldwide use of our products may be af-
fected by these cost containment pressures and cost shifting from governments and private insurers to healthcare
providers or patients in response to ongoing initiatives to reduce or reallocate healthcare expenditures. Further,
safety signals or adverse events or results from clinical trials or studies performed by us or by others (including
our licensees or independent investigators) or from the marketed use of our drugs may expand safety labeling or
restrict the use for our approved products and may negatively impact worldwide reimbursement for our products.
(See “Reimbursement.”)
We maintain sales and marketing forces primarily in the United States, Europe and Canada. We market our
products to healthcare providers including physicians or their clinics, dialysis centers, hospitals and pharmacies.
We market ENBREL under a co-promotion agreement with Wyeth in the United States and Canada (see “Joint
Ventures and Business Relationships — Wyeth”). In addition, we have entered into licensing and/or co-promotion
agreements to market our principal products in certain geographic areas outside of the United States. In the Unit-
ed States, we sell primarily to wholesale distributors of pharmaceutical products. Outside the United States, we
sell principally to hospitals and/or wholesalers depending upon the distribution practice in each country.
1