Amgen 2007 Annual Report Download - page 15
Download and view the complete annual report
Please find page 15 of the 2007 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
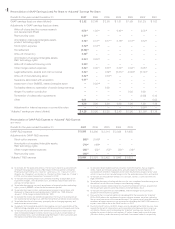
The results of the following ESA studies were released in late 2006 or during 2007:
Sponsor Study
Hb Target
(g/dL)(1) Disease Study Results
Roche(2) CREATE(3) 13-15 CKD(4) Patients with CKD of stage 3 or 4 and mild-
to-moderate anemia, the normalization of
Hb levels to 13 g/dL to 15 g/dL did not
reduce cardiovascular events as compared
with the use of a lower target range (10.5
g/dL to 11.5 g/dL)
J&J(5) CHOIR(6) 13.5 CKD Increased risk of composite events in
patients in the ESA group (death,
myocardial infarction, congestive heart
failure and stroke); No incremental
improvement in quality of life
Amgen Anemia of Cancer(7) 12-13 Non-myeloid
malignancies
Higher mortality in ESA group
DAHANCA(8) DAHANCA-10(9) 14-15.5 HNC(10) 5-year locoregional control poorer in ESA
group; No significant difference
in overall survival
AGO(11) PREPARE(9)(12) 12.5-13 Neoadjuvant
breast cancer
No significant difference in pathologic
complete remission between groups;
Decreased 3-year relapse-free and overall
survival
GOG(14) GOG-191 12-14 Cervical cancer Decreased 3-year PFS(13) and overall
survival and locoregional control
(1) Hemoglobin (“Hb”) measured in grams per deciliter (“g/dL”)
(2) F. Hoffmann-La Roche Ltd. (“Roche”)
(3) Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta (“CREATE”)
(4) Chronic kidney disease (“CKD”)
(5) Johnson & Johnson (“J&J”)
(6) Correction of Hemoglobin and Outcomes in Renal Insufficiency (“CHOIR”)
(7) Anemia of Cancer (“AoC” ‘103 study)
(8) Danish Head and Neck Cancer (“DAHANCA”)
(9) Study included as part of Aranesp®pharmacovigilance program (see “Postmarketing and Safety
Activities”).
(10) Head and neck cancer (“HNC”)
(11) German Gynecological Oncology Study Group (“AGO”)
(12) Preoperative Epirubicin Paclitaxel Aranesp®(“PREPARE”)
(13) Progression-free survival (“PFS”)
(14) Gynecologic Oncology Group (“GOG”)
The studies summarized in the table above explored the use of ESAs in settings different from those out-
lined in the FDA approved label, including targeting higher Hb levels and/or use in non-approved patient
populations. As the results of these studies were reported, various regulatory and reimbursement agencies began
to review the administration and reimbursement of ESA products resulting in certain key developments which
3