Amgen 2007 Annual Report Download - page 5
Download and view the complete annual report
Please find page 5 of the 2007 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
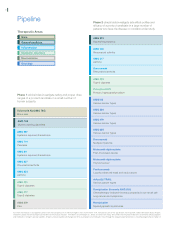
• We had an enormously productive year in research and
development, with unprecedented expansion of our early
pipeline. We introduced 13 new molecules into development,
including two phase 2 candidates from acquisitions of Ilypsa
and Alantos.
• We advanced our mid- to late-stage pipeline, including
nine phase 2 programs that we hope to progress towards
late-stage clinical trials.
• We also moved forward with denosumab, our novel
monoclonal antibody we are developing for postmenopausal
osteoporosis, bone metastases, and other serious bone loss
conditions. Early this year, we announced promising findings
from our head-to-head study comparing denosumab to
alendronate in postmenopausal women with low bone mineral
density. We look forward to communicating more study results
as we have them, including important phase 3 fracture data we
expect to review later this year.
• We recently announced an exciting new partnership in
Japan with Takeda, who will acquire rights in Japan to
VectibixTM and Japanese development rights to as many as
12 additional molecules from Amgen’s pipeline. In return, we
receive a substantial upfront fee, success-based milestones
and royalties, which we will use to advance our pipeline globally.
This collaboration validates the significant value of our
clinical pipeline and further ensures patients in Japan and around
the world will have access to our potential new medicines
for serious illness.
We know that 2008 will bring new challenges. Based on our
performance in 2007, I am confident that we can handle
whatever happens. We are ready.
KEVIN W. SHARER
Chairman and Chief Executive Officer
February 7, 2008
3
2007 Highlights
• Submitted applications in the United States, European
Union, Australia and Canada for NplateTM (romiplostim) for
the treatment of adult chronic immune thrombocytopenic
purpura (ITP), an autoimmune bleeding disorder.
• Received conditional marketing authorization from the
European Commission for VectibixTM (panitumumab) in the
European Union for use as a monotherapy in patients with
refractory metastatic colorectal cancer with non-mutated
(wild-type) KRAS genes.
• Acquired Alantos and Ilypsa, two private companies
developing drugs for the treatment of diabetes and
inflammatory diseases and for renal disorders, respectively.
Both acquisitions added novel clinical programs to
Amgen’s pipeline.
• Presented clinical data on investigational and marketed
medicines, including first-time clinical data for five phase 1
oncology programs; phase 2 and phase 3 data for
denosumab, a late-stage investigational molecule for the
potential treatment of a variety of bone loss conditions;
and new biomarker data for VectibixTM.
• Entered a collaboration and license agreement for the
development and commercialization of denosumab in
Japan with Daiichi Sankyo Company, Limited.
• Announced combination of commercial operations in
Italy with Dompé Biotec under a single company named
Amgen Dompé S.p.A.
• Gave more than $225 million through the Amgen
Foundation, Amgen’s corporate giving and product
donations. Key philanthropic programs include Amgen
Scholars, a scientific research program for university
undergraduates, and the Harold P. Freeman Patient
Navigation Institute, a national resource helping to address
health care disparities through the use of “care navigators”
who work with underserved patients.
• Named by The Scientist as one of the “Best Places
to Work in Industry.”
• Recognized for environmental sustainability programs
that target conservation of energy and resources, waste
reduction and recycling.