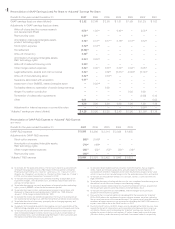
Amgen 2007 Annual Report Download - page 18
Download and view the complete annual report
Please find page 18 of the 2007 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.In particular, we indicated that we share the serious concerns voiced by physicians and their patients that one as-
pect of the Decision Memorandum, namely the restriction on reimbursement for ESAs when Hb is greater than
10 g/dL, should be reconsidered. We believe that this restriction prevents physicians from using their discretion
in appropriately managing CIA with ESAs in individual Medicare patients, subjects Medicare beneficiaries to an
untested treatment regimen and may subject them to receive otherwise avoidable blood transfusions. On De-
cember 22, 2007, we submitted to CMS a supplement to the November 13, 2007 reconsideration request to
reflect updated clinical data.
In addition to the above, further developments occurred in 2007 that have and will continue to affect the
administration and reimbursement of our ESA products. For example, on July 20, 2007, the CMS published re-
visions to its Claims Monitoring Policy: Erythropoietin/darbepoetin alfa usage for beneficiaries with end stage
renal disease (“EMP”), effective January 1, 2008, requiring a 50% reduction in Medicare reimbursement if a pa-
tient’s Hb is above 13 g/dL for three or more consecutive months. In addition, the EMP reduces the monthly
dosing limits to 400,000 international units (“IUs”) of EPOGEN®, from 500,000 IUs, and to 1,200 micrograms
(“mcgs”) of Aranesp®, from 1,500 mcgs. Also, on August 30, 2007, the National Kidney Foundation (“NKF”)
distributed to the nephrology community final Kidney Disease Outcomes Quality Initiative (“KDOQI”) clinical
practice guidelines and recommendations for anemia in CKD. Based on this review, the NKF-KDOQI™ Anemia
Work Group recommended in their 2007 Update to the NKF-KDOQI™ Anemia Management Guidelines that
physicians target Hb in the range of 11 g/dL to 12 g/dL, and also stipulated that the target not be above 13 g/dL.
Restructuring
As a result of certain of the above regulatory and reimbursement developments affecting ESAs, including
our marketed ESA products Aranesp®and EPOGEN®, and the resulting impact on our operations, on August 15,
2007, we announced a plan to restructure our worldwide operations in order to improve our cost structure while
continuing to make significant R&D investments and build the framework for our future growth. The restructur-
ing plan is also, to a lesser degree, the result of various challenges facing certain of our other products discussed
further below.
Key components of our restructuring plan include: i) staff reductions aggregating approximately 2,200 to
2,600 positions or approximately 12% to 14% of our worldwide staff, ii) rationalization of our worldwide net-
work of manufacturing facilities in order to gain cost efficiencies while continuing to meet future commercial
and clinical demand for our products and product candidates and, to a lesser degree, changes to certain R&D
capital projects and iii) abandoning leases for certain R&D facilities that will not be used in our operations. We
currently anticipate that we will incur approximately $775 million to $825 million of restructuring charges in
connection with these actions, of which $739 million was incurred through December 31, 2007.
Other regulatory developments
In addition to the developments affecting ESAs that largely led to our restructuring, there were other devel-
opments in 2007 that have negatively impacted and potentially could further impact certain of our other products.
For example, we are in discussions with the FDA with respect to the class of TNF inhibitor agents around several
safety issues, which may result in additional patient safety information in the form of a boxed warning that will
apply to the ENBREL label as has been the case with other TNF inhibitor agents.
Additionally, on March 22, 2007, as a result of safety concerns related to patient survival, we announced
that we had discontinued Vectibix™ treatment in our Panitumumab Advanced Colorectal Cancer Evaluation
(“PACCE”) trial, a non-registration-enabling trial evaluating the addition of Vectibix™ to standard chemo-
therapy and Avastin®(bevacizumab) for the treatment of first-line metastatic colorectal cancer (“mCRC”). On
October 24, 2007, we announced that we and the FDA adopted changes to the U.S. prescribing information for
Vectibix™ based on the results of the PACCE trial highlighting to clinicians the greater risk seen when Vecti-
bix™ is combined with Avastin®and the specific chemotherapy used in the PACCE trial to treat patients with
first-line mCRC. Vectibix™ is not indicated for the first-line treatment of mCRC and the new safety information
applies to an unapproved use of Vectibix™. Avastin®is the registered trademark for Genentech, Inc.’s
(“Genentech”) recombinant humanized monoclonal IgG1 antibody.
6