Amgen 2007 Annual Report Download - page 6
Download and view the complete annual report
Please find page 6 of the 2007 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.-
 1
1 -
 2
2 -
 3
3 -
 4
4 -
 5
5 -
 6
6 -
 7
7 -
 8
8 -
 9
9 -
 10
10 -
 11
11 -
 12
12 -
 13
13 -
 14
14 -
 15
15 -
 16
16 -
 17
17 -
 18
18 -
 19
19 -
 20
20 -
 21
21 -
 22
22 -
 23
23 -
 24
24 -
 25
25 -
 26
26 -
 27
27 -
 28
28 -
 29
29 -
 30
30 -
 31
31 -
 32
32 -
 33
33 -
 34
34 -
 35
35 -
 36
36 -
 37
37 -
 38
38 -
 39
39 -
 40
40 -
 41
41 -
 42
42 -
 43
43 -
 44
44 -
 45
45 -
 46
46 -
 47
47 -
 48
48 -
 49
49 -
 50
50 -
 51
51 -
 52
52 -
 53
53 -
 54
54 -
 55
55 -
 56
56 -
 57
57 -
 58
58 -
 59
59 -
 60
60 -
 61
61 -
 62
62 -
 63
63 -
 64
64 -
 65
65 -
 66
66 -
 67
67 -
 68
68 -
 69
69 -
 70
70 -
 71
71 -
 72
72 -
 73
73 -
 74
74 -
 75
75 -
 76
76 -
 77
77 -
 78
78 -
 79
79 -
 80
80 -
 81
81 -
 82
82 -
 83
83 -
 84
84 -
 85
85 -
 86
86 -
 87
87 -
 88
88 -
 89
89 -
 90
90 -
 91
91 -
 92
92 -
 93
93 -
 94
94 -
 95
95 -
 96
96 -
 97
97 -
 98
98 -
 99
99 -
 100
100 -
 101
101 -
 102
102 -
 103
103 -
 104
104 -
 105
105 -
 106
106 -
 107
107 -
 108
108 -
 109
109 -
 110
110 -
 111
111 -
 112
112 -
 113
113 -
 114
114 -
 115
115 -
 116
116 -
 117
117 -
 118
118 -
 119
119 -
 120
120 -
 121
121 -
 122
122 -
 123
123 -
 124
124 -
 125
125 -
 126
126 -
 127
127 -
 128
128 -
 129
129 -
 130
130 -
 131
131 -
 132
132 -
 133
133 -
 134
134 -
 135
135 -
 136
136 -
 137
137 -
 138
138 -
 139
139 -
 140
140 -
 141
141 -
 142
142 -
 143
143 -
 144
144 -
 145
145 -
 146
146 -
 147
147 -
 148
148 -
 149
149 -
 150
150 -
 151
151 -
 152
152 -
 153
153 -
 154
154 -
 155
155 -
 156
156 -
 157
157 -
 158
158 -
 159
159 -
 160
160 -
 161
161 -
 162
162 -
 163
163 -
 164
164 -
 165
165 -
 166
166 -
 167
167 -
 168
168 -
 169
169 -
 170
170 -
 171
171 -
 172
172 -
 173
173 -
 174
174 -
 175
175 -
 176
176 -
 177
177 -
 178
178 -
 179
179 -
 180
180
 |
 |

General medicine
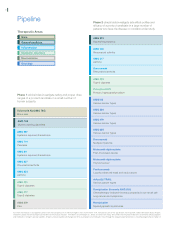
Pipeline
Phase 1 clinical trials investigate safety and proper dose
ranges of a product candidate in a small number of
human subjects.
Phase 2 clinical trials investigate side effect profiles and
efficacy of a product candidate in a large number of
patients who have the disease or condition under study.
For more information on our pipeline, please visit www.amgen.com or refer to Amgen’s most recent Form 10-K, included as part of this publication. For important safety information about Amgen
medicines, please visit www.amgen.com for links to the product websites. This table is as of February 27, 2008, and shows the status and certain next-expected milestones of selected clinical programs
and molecules in Amgen’s product pipeline. Amgen’s product pipeline will change over time as programs and molecules move through the drug development process, including progressing to market or
4
Therapeutic Areas
Inflammation
Metabolic disorders
Neuroscience
Oncology
Bone
rhApo2L/TRAIL
Various cancer types
Panitumumab
Locally advanced head and neck cancer
Motesanib diphosphate
Thyroid cancer
Romiplostim
Myelodysplastic syndromes
Romiplostim (formerly AMG 531)
Chemotherapy-induced thrombocytopenia in non-small cell
lung cancer and lymphoma
AMG 655
Various cancer types
AMG 479
Various cancer types
AMG 386
Various cancer types
AMG 102
Various cancer types
Denosumab
Multiple myeloma
Motesanib diphosphate
First-line breast cancer
AMG 223
Hyperphosphatemia
AMG 222
Type 2 diabetes
Cinacalcet HCl
Primary hyperparathyroidism
AMG 108
Rheumatoid arthritis
AMG 317
Asthma
Denosumab
Rheumatoid arthritis
AMG 379
Pain
AMG 221
Type 2 diabetes
AMG 477
Type 2 diabetes
AMG 557
Systemic lupus erythematosus
AMG 811
Systemic lupus erythematosus
AMG 714
Psoriasis
AMG 853
Asthma
AMG 827
Rheumatoid arthritis
Sclerostin Ab (AMG 785)
Bone loss
AMG 745
Muscle wasting disorders
