Johnson and Johnson 2005 Annual Report Download - page 8
Download and view the complete annual report
Please find page 8 of the 2005 Johnson and Johnson annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
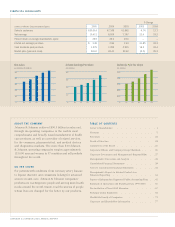
PAGE 6 JOHNSON & JOHNSON 2005 ANNUAL REPORT
Product innovations and collaboration enable physicians worldwide to transform
treatment for patients who suffer from circulatory disease.
The CARTOMERGE™ Image Integration Software Module from
Biosense Webster, Inc. is a technological breakthrough
for diagnosing arrhythmias, or abnormal rhythms that
can cause a heart to pump less effectively. Developed
in collaboration with industry leaders in imaging, the
CARTOMERGE™ Module enables electrophysiologists
to create an accurate 3-dimensional electroanatomical
map of a patient’s heart and register it with the precision
of computer tomography (CT) or magnetic resonance
imaging (MRI) scans. Merging a CT or MRI scan with
the Biosense Webster CARTO™ XP Navigation System
enables physicians to navigate and map within exact
anatomical structures of the heart, facilitating the
diagnosis and treatment of cardiac arrhythmias.
Cordis Corporation acquired LuMend, Inc., which focuses
on the development of endovascular devices to treat
chronic total occlusions (CTO) in peripheral vascular
disease. A CTO is a complete blockage of an artery
that can lead to the need for surgery or lower extremity
amputation. LuMend markets the FRONTRUNNER®
XP CTO and OUTBACK®LTD™ Re-Entry Catheter
devices that facilitate the placement of a guidewire in
minimally invasive procedures such as angioplasty
and stenting. LuMend products and technologies comple-
ment the portfolio of the Cordis Endovascular Division
of Cordis Corporation.
PALMAZ® BLUE™ Stent, launched in Europe by Cordis
Endovascular, is the latest advancement in balloon-
expandable stent technology. The PALMAZ®BLUE™
Transhepatic Biliary Cobalt Chromium and Peripheral
Stent Systems feature L605, a cobalt alloy enhanced
with tungsten, which is stronger than stainless steel
and uses less metal. It is designed to provide physicians
with increased strength, radiopacity, low profiles, and
superior flexibility and deliverability. The PALMAZ®
BLUE™ Peripheral Stent System is available in Europe
for the treatment of certain atherosclerotic lesions.
Worldwide, approximately 60 million people have peri-
pheral vascular disease (PVD), the most common disease
of the arteries, caused by a build-up of fatty substances
or plaque in the linings of bloods vessels. PVD may
cause loss of limb or death. Each year, tens of thousands
of people also are affected by life-threatening blockages
in the bile ducts, leading to the need for treatment. A
biliary stent is often used to open blockages so that fluids
may continue to pass through organs such as the liver,
gallbladder and small intestines. The PALMAZ®BLUE™
Transhepatic Biliary Cobalt Chromium Stent was cleared
in the U.S. for treating biliary ducts.
The medical technology of the CYPHER® Sirolimus-eluting
Coronary Stent, developed and manufactured by Cordis
Corporation, offers an effective, safe treatment alterna-
tive to open-heart surgery for a broad range of coronary
artery disease patients. Available in 80 countries, the
CYPHER®Stent has been used to treat more than 1.7
million patients. Significant worldwide growth for the
CYPHER®Stent in 2005 was driven by an impressive
volume of new clinical data from wide-ranging drug-
eluting stent studies that underscore its unsurpassed
long-term efficacy and trusted safety
profile. Both company-sponsored
and independently funded
studies document the success
of the CYPHER®Stent in a
variety of patient populations,
both simple and complex. These data, which contribute
to the greatest breadth of clinical data over the longest
follow-up of any drug-eluting stent, have been instru-
mental in establishing Cordis as the worldwide market
leader in drug-eluting stents.