Johnson and Johnson 2005 Annual Report Download - page 11
Download and view the complete annual report
Please find page 11 of the 2005 Johnson and Johnson annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
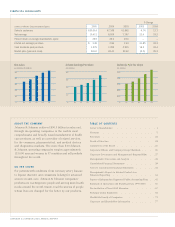
REACHING MORE PEOPLE, SERVING MORE HEALTH NEEDS. PAGE 9
The acquisition of Closure Medical Corporation has greatly
increased Ethicon, Inc.’s biomaterial-based medical
device product offerings and capabilities in topical
adhesives and surgical sealants. Closure Medical’s pro-
prietary technology can be found in DERMABOND®
Topical Skin Adhesive (2-octyl cyanoacrylate) products
and BAND-AID®Brand Liquid Bandage. Recently,
ETHICON OMNEX™ Surgical Sealant from Closure
Medical received European CE Mark approval for
use as an adjunct to sutures to achieve hemostasis,
or stop bleeding, in peripheral vascular surgery.
The Johnson & Johnson Medical (China) Ltd. Science Center,
which opened in Beijing in October 2005, is the
largest institution of its kind in the Asia Pacific region
and the company’s second medical science center in
China. The institute provides professional education and
skills training in traditional and minimally invasive
surgery, cardiovascular interventional therapy, ortho-
paedics and endocrinology, as well as an opportunity
for academic exchange among health care professionals
globally. The state-of-the-art facility is equipped with
simulators and a telesurgery system that allows surgeons
to engage in procedures from around the world. The
center is also introducing inventory and hospital man-
agement training, as well as operating room nurse
management training, to medical institutions in China.
ULTRAPRO® Synthetic Partially Absorbable Mesh from
Ethicon Products Worldwide Division of Ethicon, Inc. is
a lightweight and partially absorbable mesh for repairing
hernias and other defects of the abdominal fascia, or con-
nective tissue layer. ULTRAPRO®Mesh incorporates the
key elements of light-weight
mesh design: thin filaments,
large pore size and absorbable
components. It allows for
clear visualization of anatomy,
creates a strong, secure repair and promotes
a flexible scar that allows the abdominal wall to move
more naturally than traditional polypropylene meshes.
GYNECARE PROLIFT® Pelvic Floor Repair Systems from
Ethicon Women’s Health & Urology Division of Ethicon,
Inc. is an innovative and effective surgical option for
treating female pelvic organ prolapse, which may
occur when pelvic muscles are weakened. GYNECARE
PROLIFT®Systems are designed to reinforce tissue
and stabilize pelvic floor support structures. Until now,
traditional surgical treatments have had reported
failure rates up to 40 percent.
As surgeons perform an increasing number of complex
procedures using minimally invasive techniques, Ethicon
Endo-Surgery, Inc. understands and meets their needs
with advanced instruments. The HARMONIC ACE™
Curved Shear, the next generation of HARMONIC™
ultrasonic cutting and coagulation surgical devices from
Ethicon Endo-Surgery, enables surgeons to coagulate,
cut, grasp and dissect various tissue and blood vessels
without the need to change instruments. The ENDOPATH®
XCEL™ Trocar also facilitates precision and efficiency
during surgery by allowing the surgeon one-handed
instrument exchange and unencumbered laparoscopic
access. The ECHELON™ 60 ENDOPATH®Stapler brings
strength and precision to advanced laparoscopic pro-
cedures such as gastric bypass and colorectal surgery
by providing a long shaft for better access and the
capability to achieve hemostasis, or stop bleeding, on
thin to thick tissue. Meanwhile, the
CONTOUR™ Curved Cutter Stapler
is the only curvilinear cutter stapler
for colorectal surgery designed to fit the natural anatomy
of the body and provide improved access for surgeons.
The instrument gives surgeons the ability to gain lower
access into the pelvis than previous instruments to both
cut and staple during a lower anterior resection. The
unique curved head design also enhances the surgeon’s
visibility of anatomic structures.