Amgen 2013 Annual Report Download - page 20
Download and view the complete annual report
Please find page 20 of the 2013 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
clinical trials investigate the safety and efficacy of a product candidate in a large number of patients who have the disease or condition under study.
clinical trials investigate side effect profiles and efficacy of a product candidate in a large number of patients who have the disease or condition under study.
clinical trials investigate safety and proper dose ranges of a product candidate in a small number of human subjects.
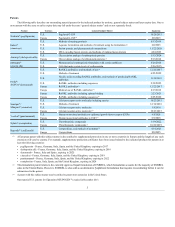
Phase 3 Product Candidate Program Changes
As of February 11, 2013, we had 14 phase 3 programs. As of February 17, 2014, we had 16 phase 3 programs, as two programs had advanced into
phase 3 trials, one program concluded and one program was added as a result of our Onyx acquisition. These changes are set forth in the following table:
Blinatumomab
ALL
Advanced to phase 3
Velcalcetide (AMG 416)
Secondary hyperparathyroidism in patients with CKD receiving dialysis
Advanced to phase 3
XGEVA®
Delay or prevention of bone metastases in prostate cancer (EU only)
Concluded - No longer pursing our marketing
application with the EMA
Kyprolis®
Multiple myeloma
Added through acquisition of Onyx
Phase 3 Product Candidate Patent Information
The following table describes our outstanding composition of matter patents that have issued thus far for our product candidates in phase 3 development
that have yet to be approved for any indication. Patents for products already approved for one or more indications but currently undergoing phase 3 clinical
trials for additional indications are previously described. See Marketing, Distribution and Selected Marketed Products — Patents.
Blinatumomab
U.S.
Polypeptides
2019
Europe
Polypeptides
2019
Brodalumab
U.S.
Polynucleotides and polypeptides
2027
Evolocumab (AMG 145)
U.S.
Polypeptides
2029
Rilotumumab
U.S.
Polypeptides
2028
Romosozumab
U.S.
Polypeptides
2026
Europe
Polypeptides
2026
Talimogene laherparepvec
U.S.
Modified HSV-1 compounds and strains
2021
Europe
Modified HSV-1 compounds and strains
2021
Trebananib
U.S.
Polynucleotides and polypeptides
2025
Europe
Polynucleotides and polypeptides
2022
Velcalcetide (AMG 416)
U.S.
Compound
2030
*Patent expiration estimates are based on issued patents which may be challenged, invalidated, or circumvented by competitors. The patent expiration
estimates do not include any term adjustments, extensions or supplemental protection certificates that may be obtained in the future and extend these
dates. Corresponding patent applications are pending in other jurisdictions. Additional patents may be filed or issued and may provide additional
exclusivity for the product candidate or its use.
Phase 3 and 2 Program Descriptions
The following text provides additional information about selected product candidates that have advanced into human clinical trials.
Aranesp®
Aranesp® is a recombinant human protein agonist of the erythropoietin receptor.
The phase 3 study of Aranesp® for the treatment of low risk myelodysplastic syndromes is ongoing.
14