Amgen 2013 Annual Report Download - page 18
Download and view the complete annual report
Please find page 18 of the 2013 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
We focus our R&D on novel human therapeutics for the treatment of grievous illness in the areas of oncology, hematology, inflammation, bone health,
nephrology, cardiovascular and general medicine, which includes neuroscience. We take a modality-independent approach to R&D with a focus on biologics.
Our discovery research programs may therefore yield targets that lead to the development of human therapeutics delivered as large molecules, small molecules,
or other combination or new modalities. For the years ended December 31, 2013, 2012 and 2011, our R&D expenses were $4.1 billion, $3.4 billion and $3.2
billion, respectively.
We have major R&D centers in several locations throughout the United States and in the United Kingdom, as well as smaller research centers and
development facilities globally. See Item 2. Properties.
We conduct clinical trial activities using both our internal staff and third-party contract clinical trial service providers. To increase the number of
patients available for enrollment in our clinical trials, we have opened clinical sites and will continue to open clinical sites and to enroll patients in a number of
geographic locations. See Government Regulation — Clinical Development and Product Approval for a discussion of government regulation over clinical
development. Also see Item 1A. Risk Factors — We must conduct clinical trials in humans before we can commercialize and sell any of our product
candidates or existing products for new indications.
Some of our competitors are actively engaged in R&D in areas where we have products or where we are developing product candidates or new indications
for existing products. For example, we compete with other clinical trials for eligible patients, which may limit the number of available patients who meet the
criteria for certain clinical trials. The competitive marketplace for our product candidates is significantly dependent on the timing of entry into the market.
Early entry may have important advantages in gaining product acceptance, thereby contributing to the product’s eventual success and profitability.
Accordingly, we expect that in some cases, the relative speed with which we can develop products, complete clinical testing, receive regulatory approval and
supply commercial quantities of the product to the market will be important to our competitive position.
In addition to product candidates and marketed products generated from our internal R&D efforts, we acquire companies, acquire and license certain
product and R&D technology rights and establish R&D arrangements with third parties to enhance our strategic position within our industry by strengthening
and diversifying our R&D capabilities, product pipeline and marketed product base. In pursuing these R&D arrangements and licensing or acquisition
activities, we face competition from other pharmaceutical and biotechnology companies that also seek to license or acquire technologies, product candidates or
marketed products from those entities performing the R&D.
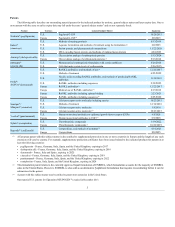
The following table is a selection of certain of our product candidates by phase of development in our therapeutic areas of focus as of February 17,
2014, unless otherwise indicated. Additional product candidate information can be found on our website at http://www.amgen.com. The website address is not
intended to function as a hyperlink, and the information contained on our website is not intended to be a part of this filing. The information in this section
does not include other, non-registrational clinical trials that we may conduct for purposes other than for submission to regulatory agencies for their approval of
a new product indication. We may conduct non-registrational clinical trials for various reasons including to evaluate real-world outcomes or to collect
additional safety information with the use of our products. In addition, the table does not include the biosimilar products we are developing, which are
discussed later in this section.
12