Amgen 2013 Annual Report Download - page 11
Download and view the complete annual report
Please find page 11 of the 2013 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
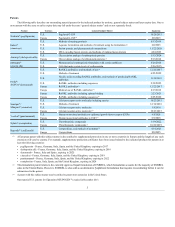
The following table describes our outstanding material patents for the indicated product by territory, general subject matter and latest expiry date. One or
more patents with the same or earlier expiry date may fall under the same “general subject matter” and are not separately listed.
Neulasta® (pegfilgrastim)
U.S.
Pegylated G-CSF
10/20/2015
Europe
Pegylated G-CSF(1)
2/8/2015
Enbrel®
(etanercept)
U.S.
Methods of treating psoriasis
8/13/2019
U.S.
Aqueous formulation and methods of treatment using the formulation (2)
6/8/2023
U.S.
Fusion protein, and pharmaceutical compositions
11/22/2028
U.S.
DNA encoding fusion protein, and methods of making fusion protein
4/24/2029
Aranesp® (darbepoetin alfa)
U.S.
Glycosylation analogs of erythropoietin proteins
5/15/2024
Europe
Glycosylation analogs of erythropoietin proteins (1)
8/16/2014
EPOGEN®
(epoetin alfa)
U.S.
Pharmaceutical erythropoietin formulation with certain stabilizers
8/26/2014
U.S.
Cells that make certain levels of erythropoietin
5/26/2015
Prolia®/
XGEVA® (denosumab)
U.S.
RANKL antibodies; and methods of use(3)
12/22/2017
U.S.
Methods of treatment
6/25/2022
U.S.
Nucleic acids encoding RANKL antibodies, and methods of producing RANKL
antibodies
11/30/2023
U.S.
RANKL antibodies including sequences
2/19/2025
Europe
RANKL antibodies(1)
12/22/2017
Europe
Medical use of RANKL antibodies(1)
4/15/2018
Europe
RANKL antibodies including epitope binding
2/23/2021
Europe
RANKL antibodies including sequences(1)
6/25/2022
Sensipar®/
Mimpara® (cinacalcet)
U.S.
Calcium receptor-active molecules including species
10/23/2015
U.S.
Methods of treatment
12/14/2016
U.S.
Calcium receptor-active molecules
3/8/2018
Europe
Calcium receptor-active molecules(1)
10/23/2015
Vectibix® (panitumumab)
U.S.
Human monoclonal antibodies to epidermal growth factor receptor (EGFr)
4/8/2020
Europe
Human monoclonal antibodies to EGFr(1)
5/5/2018
Nplate® (romiplostim)
U.S.
Thrombopoietic compounds
1/19/2022
Europe
Thrombopoietic compounds(1)
10/22/2019
Kyprolis® (carfilzomib)
U.S.
Compositions, and methods of treatment(3)
4/14/2025
Europe
Compositions
8/8/2025
(1) A European patent with this subject matter is also entitled to supplemental protection in one or more countries in Europe and the length of any such
extension will vary by country. For example, supplementary protection certificates have been issued related to the indicated products for patents in at
least the following countries:
•pegfilgrastim - France, Germany, Italy, Spain, and the United Kingdom, expiring in 2017
•darbepoetin alfa - France, Germany, Italy, Spain, and the United Kingdom, expiring in 2016
•denosumab - France, Italy and Spain, expiring in 2025
•cinacalcet - France, Germany, Italy, Spain, and the United Kingdom, expiring in 2019
•panitumumab - France, Germany, Italy, Spain, and the United Kingdom, expiring in 2022
•romiplostim - France, Italy, Spain, and the United Kingdom, expiring in 2024
(2) This formulation patent relates to the currently approved liquid formulation of ENBREL, which formulation accounts for the majority of ENBREL
sales in the United States. However, ENBREL is also sold as an alternative lyophilized formulation that requires reconstituting before it can be
administered to the patient.
(3) A patent with this subject matter may be entitled to patent term extension in the United States.
Our material U.S. patents for filgrastim (NEUPOGEN ®) expired in December 2013.
5