Johnson and Johnson 2009 Annual Report Download - page 12
Download and view the complete annual report
Please find page 12 of the 2009 Johnson and Johnson annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
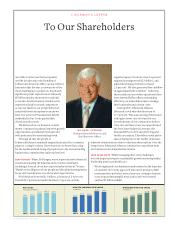
10 JOHNSON & JOHNSON 2009 ANNUAL REPORT
“Everything happened so fast,
and there wasn’t much
information: ‘You have cancer,
you’ll have your surgery and
then radiation’—it was
a generic recipe for a patient
with breast cancer,” says
Jesica Harrington, now
and a breast cancer survivor.
to detect changes in a patient’s status and
provides information about the person’s
prognosis, to help physicians make more
informed patient care decisions.
“I asked my oncologist, if it was as easy as
getting my blood drawn, to please order
this test and I’d be forever grateful,” says
Jesica. “When the first test came back and it
was one cell—and anything less than five is
a good prognosis—that gave me hope.”
The ® System is the first
diagnostic test that automates the capture
and detection of CTCs, tumor cells that
have detached from solid tumors and
entered the patient’s blood. It assesses
CTCs to determine the prognosis and
overall survival of patients with
metastatic breast, colorectal or
prostate cancer at any time during the
course of treatment.
The Cleveland Clinic ranked the
technology used in the ®
System as the top medical innovation for
, predicting it will have a significant
impact on health care. And in September,
the ® CTC test was honored
with the first-ever Prix Galien USA Award
for Best Medical Technology. The Prix
Galien recognizes technical, scientific
and clinical research skills necessary to
develop innovative medicines and
devices, and is considered the industry’s
highest accolade. Candidates are evaluated
on the innovative nature of their develop-
ment and applicability of the technology,
and on whether the discovery can be
applied to future biomedical science.
“The future
goes beyond counting cancer cells toward
using the ® platform to
characterize individual patients’ tumors by
gathering data about protein
and genetic
markers,” says David Chianese,
Principle
Scientist, Cellular Research, Veridex, LLC.
“Our progress in this area has been driven
by drug development needs and oncology
trials.”
The ® platform is used in
a Joint Venture Convergence Project that
involves Veridex, LLC and Centocor
Ortho Biotech, Inc., an oncology
pharmaceutical area of business. It is also
used in important academic collabora-
tions, such as with Royal Marsden
Hospital in London and Memorial Sloan-
Kettering Cancer Center in New York. The
two are clinical study sites for abiraterone
acetate, a late-stage, first-in-class
compound for the treatment of prostate
Jesica was pregnant with twins when
she received her diagnosis in November
. A month later, she lost one of the
babies and underwent a mastectomy
before beginning four rounds of
chemotherapy. Jesica simply wanted to
know how her doctors would determine
whether the treatment was fighting her
cancer. “I told them hope and prayer was
just not enough for me,” she recalls.
Because Jesica was pregnant, her
oncologist couldn’t use the usual methods
of imaging to get diagnostic information
or details about her prognosis once
beginning treatment. Jesica’s father
understood his daughter’s need for
information to fuel her hope of beating
the cancer. On the Internet, he found out
about the ® Circulating
Tumor Cell (CTC) test from Veridex
, LLC.
This simple blood test captures,
identifies and counts circulating tumor
cells in patients with certain types of
metastatic cancer. When used in
combination with other tests, the
® CTC test allows a doctor
The CELLSEARCH® Circulating Tumor Cell test
is not approved to demonstrate that any current
line of therapy is any more or less eective
than any other or no therapy. CELLSEARCH®
results and imaging results are not equivalent
in assessing the transition of patients between
non-progressive disease and progressive
disease.
A simple test allows doctors to personalize care.