Eli Lilly 2012 Annual Report Download - page 7
Download and view the complete annual report
Please find page 7 of the 2012 Eli Lilly annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
5
Last August, we announced top-line results from two
Phase III trials of solanezumab in patients with mild-to-
moderate Alzheimer’s disease. While primary endpoints,
both cognitive and functional, were not met in either
trial, a pre-specied secondary analysis of pooled data in
patients with mild Alzheimer’s disease showed a statisti-
cally signicant 34 percent slowing of decline in the
treated group compared to those who
received placebo.
In December, we announced that
we plan to conduct an additional Phase
III study of solanezumab in patients
with mild Alzheimer’s disease, which
we expect to initiate no later than
the third quarter of this year. We are
excited about the solanezumab results,
and we will continue to analyze and
discuss the data from the two com-
pleted studies with global regulators.
(See page 8 for more information.)
In October, Lilly announced posi-
tive top-line results of three completed
Phase III trials for dulaglutide, our
GLP-1 analog being studied as a once-
weekly treatment for type 2 diabetes.
Dulaglutide demonstrated statistically
superior reductions in HbA1c com-
pared to exenatide twice-daily injection
at 26 weeks; metformin at 26 weeks; and sitagliptin at
52 weeks.
Baricitinib entered Phase III testing in the fall of
2012 after Lilly and our partner Incyte announced
positive results of a Phase IIb trial in patients with active
rheumatoid arthritis. In addition, The New England Jour-
nal of Medicine published results of the positive Phase II
trial in psoriasis of our anti-IL-17 monoclonal antibody
ixekizumab.
In January 2013, Lilly and Boehringer Ingelheim
announced topline results for four completed Phase III
clinical trials for empagliozin, an investigational SGLT-
2 inhibitor for type 2 diabetes. In all four studies, the
primary ecacy endpoint—dened as change in HbA1c
from baseline compared to placebo—was met with
empagliozin 10 mg and 25 mg taken once daily.
Finally, early this year we presented the rst Phase
III data for ramucirumab, our VEGFR-2 antibody from
ImClone. As a single agent in patients with second-line
metastatic gastric cancer—for which there are now no
treatments specically approved in the U.S. and Europe—
ramucirumab met its primary endpoint of improved
overall survival and also showed prolonged progression-
free survival. We intend to submit for regulatory approval
in the U.S. and Europe in 2013.
Reafrming Our Strategy and Commitment
The data disclosed this past year—notably Phase III
data on solanezumab, ramucirumab, dulaglutide, and empa-
gliozin, as well as Phase II data on baricitinib, ixekizumab,
and our novel basal insulin—rearm our condence in, and
commitment to, our innovation-based strategy.
During 2013, we expect to have
new data from Phase III trials—much
of which will be presented at scientic
meetings—on eight of our 13 Phase III
assets. We also anticipate submitting as
many as ve Phase III candidates for
regulatory approval. Potential lings
include three in diabetes—dulaglutide,
empagliozin, and our new insulin
glargine product—and two in oncol-
ogy—ramucirumab as monotherapy
for second-line gastric cancer, as well
as enzastaurin.
In sum, while there is a lag
between our patent expirations and the
next wave of new product launches, we
have built the strongest pipeline in our
history. We believe our focus on the
pipeline represents the right path for
Lilly, and our recent performance gives
us increasing condence in our ability
to execute our innovation strategy, to navigate through this
challenging period, and to return to growth in the years ahead.
I am proud of what Lilly people and our many partners
accomplished together in 2012, and I’m excited about our
prospects for 2013 and beyond. While there remain plenty
of challenges ahead, we clearly have the wherewithal to meet
and overcome these challenges.
My condence in saying this begins with our Lilly
people. I have the good fortune to work alongside thousands
of talented and dedicated colleagues. I especially want to
recognize and thank two members of our Executive Commit-
tee who retired at the end of 2012—Bob Armitage and Anne
Nobles—for their combined 35 years of outstanding service
to our company.
I hope that you, our Lilly shareholders, will take pride
in the continued progress of your company in pursuit of
our mission to help people live longer, healthier, more active
lives. I thank you for your support.
For the Board of Directors,
John C. Lechleiter
Chairman, President, and Chief Executive Ocer
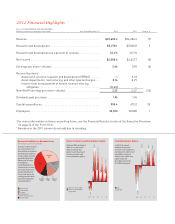
Revenue Per Employee
($ thousands, percent growth)
In 2012, revenue per
employee decreased
8 percent to $590,000, due
primarily to a 7 percent
decrease in revenue as the
result of the Zyprexa
patent loss.
08 09 10 11 12
$540 +7%
$504 +10%
$590 -8%
$602 +11%
$638 +6%