Eli Lilly 2012 Annual Report Download - page 6
Download and view the complete annual report
Please find page 6 of the 2012 Eli Lilly annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
4
rst-line Alimta plus cisplatin for locally advanced or
metastatic nonsquamous non-small cell lung cancer. We
gained new indications for Cymbalta, Zyprexa, Strattera®,
and Erbitux in Japan. And the EC approved Cialis® for
once-daily use for benign prostatic hyperplasia (BPH), or
enlarged prostate.
In addition, the U.S. Court of Appeals upheld Alimta’s
compound patent expiring January
2017, and we gained an additional
six months of market exclusivity for
Cymbalta in the U.S.—to December
2013—after meeting the FDA’s
requirements for pediatric exclusivity.
A further source of revenue
growth came from three important
growth areas—emerging markets,
Japan, and Elanco Animal Health.
In 2012, China led emerging mar-
kets with growth of 27 percent, mostly
from increased volume. Overall,
emerging markets revenue declined
4 percent—following 10 percent
growth in 2011—due mainly to patent
expirations in key countries like Brazil
and Mexico. Excluding generic entry
and the impact of foreign exchange,
emerging markets growth was
11 percent.
In Japan, revenue grew 7 percent in 2012—despite
government-mandated price cuts that trimmed our
revenue growth by 7 percent.
Elanco revenue increased by 21 percent—growing
at more than three times the rate of the animal health
industry—and crossed $2 billion in revenue for the
rst time. Five key acquisitions over the past ve years,
including Janssen Animal Health in 2011 and ChemGen
in 2012, have augmented strong internal growth, while
serving to expand our presence in Europe and establish a
foothold in vaccines.
Increasing Productivity
The negative impact of our patent expirations, coupled
with challenging market conditions, requires us to
address our cost structure, both to weather YZ and to
position our company for long-term success.
After cutting projected costs by more than $1 billion
between 2009 and 2011, we aim to reduce our costs
further and to improve productivity across the enterprise.
We’ve taken signicant steps to rein in discretionary
spending and to change the way we operate—includ-
ing, for example, reorganizing our business in Europe
in 2012. We’ll continue to use Six Sigma—which has
produced a cumulative value of $3 billion since 2005—
and other tools to drive further eciencies.
In 2012, as a result of our cost-containment eorts,
total operating expenses—the sum of R&D and market-
ing, selling, and administrative (MS&A) expenses—
declined 1 percent. A reduction of 5 percent in MS&A
expenses oset increased R&D expenses, which rose
5 percent to 23 percent of total
revenue due to late-stage clinical trial
costs.
Following the YZ period, we
expect to return to levels of R&D
spending more consistent with our
historical averages, in the range of
18 percent to 20 percent of revenue.
Further, within a few years after 2014,
we expect that MS&A expenses will
move more into line with industry
averages, in the range of 28 to 30
percent of revenue.
Advancing the Pipeline
Even as we focus on generating new
revenue and controlling expenses to
compensate for patent losses, our top
priority is advancing our pipeline. We
continue to make good progress. (See
page 10.)
In 2005, we had a total of seven assets in Phase II
and Phase III. Today, we have 13 molecules in Phase III
alone—11 of which originate in our own laboratories,
including ImClone—and nearly two dozen additional
molecules in Phase II, comprising a good mix of both
small molecules and biologics.
Our Phase III portfolio includes:
• two candidates in neuroscience—solanezumab
and edivoxetine—after Phase III development of
pomaglumetad methionil for schizophrenia was
terminated in midyear;
• four in diabetes—dulaglutide, empagliozin, our new
insulin glargine product, and our novel basal insulin;
• three in oncology—ramucirumab, necitumumab, and
enzastaurin;
• three autoimmune candidates—the oral JAK1/JAK2
inhibitor baricitinib, which moved into Phase III in
late 2012, along with ixekizumab and tabalumab;
• and evacetrapib, our CETP inhibitor, for which a
Phase III trial in high-risk vascular disease was initi-
ated in 2012.
Let me briey review progress in 2012 for several of
these potential medicines:
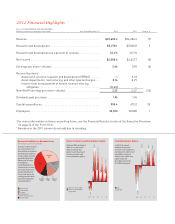
Key Growth Contributors to 2012 Revenue
($ in millions represent growth in revenue,
percent growth)
Four products and a product
line—Cymbalta, Forteo,
Effient, Alimta, and Animal
Health—generated revenue
growth of approximately
$1.7 billion during 2012 over
2011. This growth was driven
primarily by volume increases.
Cymbalta
Total Animal Health
Forteo
Effient
Alimta
$154.7 +51%
$201.2 +21%
$832.3 +20%
$357.6 +21%
$133.2 +5%