Eli Lilly 2007 Annual Report Download - page 4
Download and view the complete annual report
Please find page 4 of the 2007 Eli Lilly annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
2
LETTER TO SHAREHOLDERS
To Our Shareholders
Eli Lilly and Company delivered outstanding results in
2007, highlighted by 14 percent pro forma sales growth,
17 percent growth in pro forma adjusted net income, and
the movement of no fewer than 16 new molecules into
clinical testing. Measured more broadly by the growth
of our recently launched products in diverse markets,
the success of our business development efforts, and the
progress of discoveries through our development pipe-
line, 2007 was one of the most thoroughly successful
years in Lilly’s history.
Just as importantly in 2007, we asserted a new vision
of the company—dedicated to improving outcomes for in-
dividual patients—and we accelerated the transformation
of Lilly to realize this aspiration.
In this letter, we look forward to reporting on all of
these accomplishments in more detail.
Leadership Transition
Near the end of 2007, we announced a leadership
transition between us that had been carefully planned
for some time. Sidney Taurel will retire as chief executive
offi cer at the end of March 2008, turning over that role to
John Lechleiter. Taurel will remain chairman of the board
until the end of 2008.
Lechleiter assumes his new role as well prepared as any
of his nine predecessors at the helm of Eli Lilly and Com-
pany. A Ph.D. chemist who joined Lilly in 1979 in process
research and development (R&D), Lechleiter will redouble
Lilly’s commitment to develop fi rst-in-class and best-in-class
products. He also brings extensive experience in product
development, regulatory affairs, and global operations.
Already, he has held the reins as operational leader of Lilly
for more than two years, a period of solid sales growth and
steady performance in R&D, manufacturing, and marketing.
Our partnership in leading Lilly during the past two
years has been close and fruitful, and the nature of our
transition demonstrates a broader unity of purpose inside
the company. Lilly’s vision of delivering optimal outcomes
for individual patients is not “Sidney’s” or “John’s.” It is a
vision that we share and one that embodies the promise
of new science, the realities of the external environment,
and the aspirations of our Lilly colleagues worldwide.
We also share a belief that the Lilly CEO’s most im-
portant role is to continuously strengthen the company’s
founding values of excellence, integrity, and respect for
people. In view of the expectations and scrutiny directed at
our industry today, it has never been more important for ev-
eryone at Lilly to live up to these standards, demonstrating
their best performance as well as conduct beyond reproach.
Financial Results
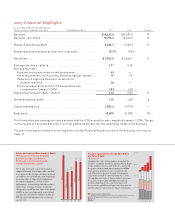
In 2007, Lilly sales increased 19 percent on a reported
basis, to $18.634 billion. On a pro forma basis, which as-
sumes we owned ICOS in both 2006 and 2007, our sales
increased 14 percent, to $18.706 billion. Our sales growth
outpaced the overall pharmaceutical sector in each of the
world’s largest markets—the U.S., Europe, and Japan—
and we achieved a 7 percent increase in sales volume
worldwide on a pro forma basis.
Demonstrating our continued commitment to grow
sales faster than operating expenses, pro forma adjusted
net income and earnings per share (EPS) both grew by
17 percent in 2007, to $3.863 billion and $3.54, respec-
tively. On a reported basis, the growth was 11 percent, to
$2.953 billion and $2.71. The pro forma adjusted results
assume we owned ICOS for both years and also adjust for
product liability expenses, asset impairments, and restruc-
turing in both years as well as for charges related to the
acquisition of compounds in development in 2007 (for a
reconciliation, see page 1).
Product Performance
Lilly’s top-selling product, Zyprexa, is beginning
to face competition from generic formulations in some
countries—notably Canada and Germany—but overall
demand for this neuroscience therapy grew in markets
outside the United States during 2007, helping to drive
worldwide sales up 9 percent to $4.761 billion. In the
U.S., a longstanding downward trend in Zyprexa’s share
of new retail prescriptions continued to slow in 2007,
in spite of new competitive products, label changes, and
negative advertising by trial lawyers.
Wider loss of patent protection on Zyprexa and other
products early in the next decade heightens the impor-
tance of strong sales growth among our newer products—
and they have risen to the challenge. Pro forma sales of
Lilly products launched in this decade collectively grew
by 33 percent in 2007 and represented 32 percent of our
total sales, up from 28 percent in 2006.
Cymbalta remains of particular importance to Lilly’s
overall performance in the years ahead, and we are
pleased that its success in 2007 is essentially unquali-
EgdYjXihAVjcX]ZYI]^h9ZXVYZ8dcig^WjiZY
+#%7^aa^dc^cHVaZh9jg^c\'%%,
*À^ÒSÍÄÊ:ÒSg^ÊÍÄÊ^gS:^gÊSÒ^gÊÛJ:Í:[Ê
-ÍÀ:ÍÍgÀ:[ÊÍ:[ÊÀÍg[Ê6zÀÄ[Ê:Ä[Ê
-ÛJÛ:Ú[ÊÛgÍÍ:[Ê:^Ê7gÍÀgØg®Ê.gÄgʨÀ^ÒSÍÄÊ
SÍÀJÒÍg^ÊdÈ®ßÊJÊÍÊgÍÊÄ:gÄÊ:^Ê
SÍÒg^ÊÍÊ^ØgÀÄsÛÊÒÀʨÀÍs®Ê.Í:Ê
ÙÀ^Ù^gÊ:ÄʨÀ^ÒSÍÊÄ:gÄÊ:ÀgÊSÒ^g^Ê
ÄSgÊÒÀÊ:Ò:ÀÛÊÑ[ÊÑßßÇÊ:SµÒÄÍÊsÊ!-®
EgdYjXihAVjcX]ZYI]^h9ZXVYZ
OnegZmV
6aaDi]Zg
'%%,
'%%+
)-
'-
')
)'
'+
('