Eli Lilly 2007 Annual Report Download - page 13
Download and view the complete annual report
Please find page 13 of the 2007 Eli Lilly annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
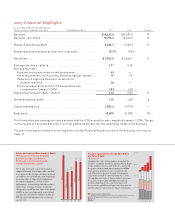
FINANCIALS
11
prasugrel versus clopidogrel in November.
• In January 2008, the FDA approved Cialis® for once-
daily use to treat erectile dysfunction. Cialis was
approved by the European Commission for once-daily
use in June 2007.
• In November, the FDA approved Cymbalta for the
maintenance treatment of major depressive disorder in
adults. In February, the FDA approved Cymbalta for the
treatment of generalized anxiety disorder. During 2007,
we submitted a Supplemental New Drug Application
to the FDA for Cymbalta for the management of
fi bromyalgia.
• In October, with our collaboration partners Amylin
Pharmaceuticals, Inc., and Alkermes, Inc., we
announced positive results from a 30-week comparator
study of once-weekly exenatide long-acting release
injection and Byetta® (exenatide) injection taken twice
daily in patients with type 2 diabetes.
• In the second quarter, we submitted NDAs to the
FDA and the European Medicines Agency (EMEA) for
approval of olanzapine (Zyprexa) long-acting injection.
• In September, the FDA approved Evista® for a new
use to reduce the risk of invasive breast cancer
in two populations: postmenopausal women with
osteoporosis and postmenopausal women at high risk
for invasive breast cancer.
• We submitted an application to the EMEA for
centralized review of Alimta®, in combination with
cisplatin, for the fi rst-line treatment of non-small cell
lung cancer.
Business Development
• In December, we entered into a licensing and
development agreement with BioMS Medical Corp.
whereby we acquired exclusive worldwide rights to
a multiple sclerosis (MS) compound. The compound
is currently being evaluated in two pivotal Phase III
clinical trials in secondary progressive MS (SPMS) and
one Phase II clinical trial in relapsing-remitting MS
(RRMS). In connection with this agreement, we will
incur a charge to earnings for acquired IPR&D of $87.0
million (pretax), which will be included as expense in
the fi rst quarter of 2008.
• In October, we entered into an agreement with
Glenmark Pharmaceuticals Limited India whereby we
acquired the rights to a portfolio of transient receptor
potential vanilloid sub-family 1 (TRPV1) antagonist
molecules, including a clinical-phase compound. The
compound is currently in Phase II development as a
potential next-generation treatment for various pain
conditions, including osteoarthritic pain.
• In October, we entered into a global strategic alliance
with MacroGenics, Inc., to develop and commercialize
teplizumab, a humanized anti-CD3 monoclonal
antibody, as well as other potential next-generation
anti-CD3 molecules for use in the treatment of
autoimmune diseases. As part of the arrangement,
we acquired the exclusive rights to the molecule.
Teplizumab is currently being studied in the PROTÉGÉ
trial, a global pivotal Phase II/III clinical trial for
individuals with recent-onset type 1 diabetes.
• In June, we completed the acquisition of Ivy Animal
Health, Inc., a privately held applied research and
pharmaceutical product development company focused
on the animal health industry. The acquisition provides
us with product lines that complement those of our
animal health business.
• In April, we completed the acquisition of Hypnion,
Inc., a privately held neuroscience drug discovery
company focused on sleep disorders. The deal expands
our presence in the area of sleep disorder research
and provides ownership of a novel Phase II insomnia
compound with a dual mechanism of action aimed at
promoting better sleep onset and sleep maintenance.
• In January, we completed the acquisition of ICOS at
a cost of approximately $2.3 billion. The acquisition
brings the full value of Cialis to us and enables us
to realize operational effi ciencies in the further
development, marketing, and selling of this product.
• In January, we licensed from OSI Pharmaceuticals its
glucokinase activator (GKA) program for the treatment
of type 2 diabetes, including the lead compound. Lilly
received an exclusive license to develop and market
any compounds derived from the GKA program.
LEGAL, REGULATORY, AND OTHER MATTERS
In October, the United States Supreme Court denied the
petitions for certiorari that were fi led by Teva Phar-
maceuticals and Dr. Reddy’s Laboratories, bringing to
an end the two companies’ challenges to the validity of
Lilly’s U.S. Zyprexa patent.
In June, we received notice of two court rulings
by the Canadian Federal Court and the German Pat-
ent Court that permit the entry of generic olanzapine
(Zyprexa) by competitors into the Canadian and German
markets. Generic olanzapine is now available for sale
by competitors in Canada and Germany.
We have reached agreements with claimants’
attorneys involved in U.S. Zyprexa product liability
litigation to settle a total of approximately 31,200 claims
against us relating to the medication. Approximately
1,235 claims remain. As a result of our product liability
exposures, since the beginning of 2005, we have re-
corded aggregate net pretax charges of $1.61 billion for
Zyprexa product liability matters.
In March 2004, we were notifi ed by the U.S. Attor-
ney’s offi ce for the Eastern District of Pennsylvania
(EDPA) that it had commenced an investigation relating
to our U.S. marketing and promotional practices for
Zyprexa, Prozac®, and Prozac Weekly™. In November
2007, we received a grand jury subpoena from the EDPA