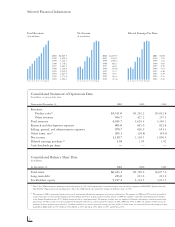
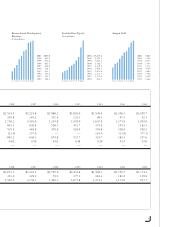
Amgen 2001 Annual Report Download - page 6
Download and view the complete annual report
Please find page 6 of the 2001 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Neulasta
™
(pegfilgrastim), Amgen’s new
white blood cell stimulator, is less-frequently
administered than NEUPOGEN
®
(Filgrastim), Amgen’s breakthrough
infection-fighting drug therapy introduced
in 1991 to support cancer patients receiving
chemotherapy. With its easier, once-per-
chemotherapy-cycle dosing, Neulasta
™
has
the potential to help more cancer patients
than ever before successfully tolerate a
complete course of chemotherapy by avoid-
ing the potential complications of infection.
Last year, we submitted U.S., European,
Canadian, and Australian applications for
its use in cancer chemotherapy treatment
settings. In January 2002, we received
U.S. approval for Neulasta
™
in the chemo-
therapy-induced neutropenia setting.
Amgen’s research and development
programs were also productive last year
in identifying and advancing a series of
potential new therapeutics. We initiated
phase 3 clinical studies for two promising
drug candidates, KGF and AMG 073. And,
more recently, we announced promising
new research collaboration agreements with
three companies. Overall, Amgen is poised
to introduce more products into develop-
ment in 2002 and 2003 than we have in
the past ten years combined. To focus our
resources more efficiently, we also chose
to end collaboration agreements with
two companies, Praecis Pharmaceuticals
Incorporated and Guilford Pharmaceu-
ticals Inc.
We continued to meet patient demand for
our existing product line, maintaining an
industry-leading position in the manufac-
turing and distribution of biologically
based human therapeutics.
All told, Amgen manufactured six
therapeutics last year, once again passing
rigorous regulatory inspections for both
quality and safety at our manufacturing
facilities. In addition, the groundwork was
laid to expand our manufacturing capacity
to meet anticipated demand for both new
and existing products. Our plans include
a significant addition to Amgen’s current
manufacturing facilities in Puerto Rico.
Organizationally, we attracted significant
new talent to key areas of our business
as we scale up Amgen to meet the chal-
lenges of a growing product line and
an increasingly ambitious research and
development program.
We’ve added new senior management to
the company’s leadership ranks in a number
of areas. Roger Perlmutter joined Amgen
as executive vice president, research and
development; Beth Seidenberg as senior
vice president, development; George
Morrow as executive vice president, world-
wide sales and marketing; Richard Nanula
as executive vice president, finance, strategy
and communications, and chief financial
officer; and Brian McNamee as senior
vice president, human resources. These
additions bolster what I believe is the
most talented and committed management
team in the biotechnology industry today.
Early this year, we were delighted to have
Patricia Sueltz, executive vice president
4