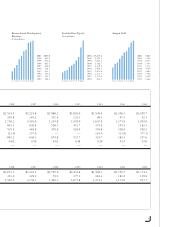
Amgen 2001 Annual Report Download - page 3
Download and view the complete annual report
Please find page 3 of the 2001 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
ultimate goal – global leadership in the
treatment of anemia in all medical settings.
Advancing that goal further were the
worldwide regulatory filings we submitted
for the use of Aranesp
™
in the treatment of
chemotherapy-induced anemia. Given the
range of conditions that can induce anemia,
and the growing body of evidence that
its early identification and treatment may
enhance overall patient outcomes, we
believe the market for anemia treatments
will approach $10 billion by 2005.
Kineret
™
(anakinra), the first therapeutic
delivered from our inflammation program,
received U.S. approval in 2001 for use in
the treatment of the signs and symptoms
of rheumatoid arthritis. Kineret
™
is unique
in its ability to mitigate inflammation by
blocking interleukin-1, a key cytokine
implicated in the immune system’s inflam-
mation cascade. We believe the worldwide
market for biologic treatments for rheuma-
toid arthritis and related diseases will
exceed $8 billion by 2005.
1
Kevin Sharer CHAIRMAN AND CHIEF EXECUTIVE OFFICER
Letter to Stockholders
The first year of the new century marked
a transition point for Amgen in several
important ways. We launched two signifi-
cant new products. We made promising
advances in clinical and preclinical research.
We brought new leadership into key areas
of our business. We announced an acquisi-
tion with great promise. And we affirmed
our core values and began to transform
key operating processes to prepare for a
more competitive and demanding future.
We are confident these steps will result in
increased value for patients and stockholders
over time.
Among the most important achievements
of the year, we established the means to
serve more patients in more ways than ever
before, launching two new products and
laying the groundwork for a third approval
achieved in early 2002.
Aranesp
™
(darbepoetin alfa) represents
a new standard of care for anemia. Its
approval in 2001 in the United States,
Europe, Australia, and New Zealand for
the treatment of anemia in chronic renal
failure is a significant step toward Amgen’s