Pfizer 2005 Annual Report Download - page 44
Download and view the complete annual report
Please find page 44 of the 2005 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
2005 Financial Report 43
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
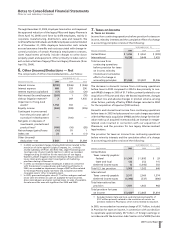
Allocation of Pharmacia Purchase Price
The purchase price allocation, finalized in the early part of 2004,
was based on an estimate of the fair value of assets acquired and
liabilities assumed.
(MILLIONS OF DOLLARS) AMOUNT
Book value of net assets acquired $ 8,795
Less: Recorded goodwill and other intangible assets 1,559
Tangible book value of net assets acquired 7,236
Remaining allocation:
Increase inventory to fair value 2,939
Increase long-term investments to fair value 40
Decrease property, plant and equipment to fair value (317)
Record in-process research and development charge 5,052
Record identifiable intangible assets(a) 37,066
Increase long-term debt to fair value (370)
Increase benefit plan liabilities to fair value (1,471)
Decrease other net assets to fair value (477)
Restructuring costs(b) (2,182)
Tax adjustments(c) (12,947)
Goodwill(a) 21,403
Purchase price $ 55,972
(a) See Note 12, Goodwill and Other Intangible Assets.
(b) See Note 5, Merger-Related Costs.
(c) See Note 7, Taxes on Income.
Since our interim allocation in the fourth quarter of 2003, the
significant revisions to our estimates relate primarily to fixed
assets ($756 million decrease), identifiable intangible assets ($155
million decrease) and tax adjustments ($645 million decrease). In
addition, in 2004, we recorded an additional $604 million in
restructuring charges as a component of the purchase price
allocation.
The more significant revisions to our estimates relating to our
initial allocation of the purchase price in the second quarter of
2003 include inventory ($1.3 billion increase), fixed assets ($1.1
billion decrease), identifiable intangible assets ($560 million
increase) and tax adjustments ($986 million decrease). In addition,
we recorded an additional $1.4 billion in restructuring charges.
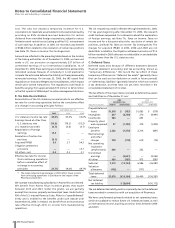
Pro Forma Results of Pharmacia Acquisition
The following unaudited pro forma financial information presents
the combined results of operations of Pfizer and Pharmacia as if
the acquisition had occurred as of the beginning of 2003. The
unaudited pro forma financial information is not necessarily
indicative of what our consolidated statement of income actually
would have been had we completed the acquisition at the
beginning of the year. In addition, the unaudited pro forma
financial information does not attempt to project the future
results of operations of the combined company.
YEAR ENDED DEC. 31,
________________________
(MILLIONS OF DOLLARS, EXCEPT PER COMMON SHARE DATA) (UNAUDITED) 2003
Revenues $48,292
Income from continuing operations before
cumulative effect of a change in accounting
principles 8,265
Net income 10,536
Per share amounts:
Income from continuing operations before
cumulative effect of a change in accounting
principles per common share—basic 1.06
Net income per common share—basic 1.36
Income from continuing operations before
cumulative effect of a change in accounting
principles per common share—diluted 1.05
Net income per common share—diluted 1.34
The unaudited pro forma financial information above reflects the
following:
•The elimination of transactions between Pfizer and Pharmacia,
which upon completion of the merger would be considered
intercompany. The majority of these transactions occurred
under the Celebrex and Bextra marketing agreements. This
reflects:
•the elimination of certain sales, alliance revenue and certain
co-promotion expenses
•the elimination of certain impacts of milestone payments
made by Pfizer to Pharmacia
•A decrease in interest expense of $11 million related to the
estimated fair value adjustment of long-term debt from the
purchase price allocation.
•Additional amortization and depreciation expense of
approximately $993 million related to the estimated fair value
of identifiable intangible assets and property, plant and
equipment from the purchase price allocation.
The unaudited pro forma financial information above excludes the
following material, non-recurring charges incurred in the year
ended December 31, 2003:
•Purchase accounting adjustments related to a charge for IPR&D
of $5.1 billion and the incremental charge of $2.7 billion
reported in Cost of sales for the sale of acquired inventory that
was written up to fair value.
B. Other Acquisitions
On September 14, 2005, we completed the acquisition of all of the
outstanding shares of Vicuron Pharmaceuticals, Inc. (Vicuron), a
biopharmaceutical company focused on the development of
novel anti-infectives, for approximately $1.9 billion in cash
(including transaction costs). At the date of acquisition, Vicuron
had two products under NDA review by the U.S. Food and Drug
Administration (FDA): Eraxis (anidulafungin) for fungal infections
and Zeven (dalbavancin) for Gram-positive infections. The
allocation of the purchase price includes IPR&D of approximately
$1.4 billion, which was expensed in Merger-related in-process
research and development charges, and goodwill of $243 million,