Eli Lilly 2011 Annual Report Download - page 6
Download and view the complete annual report
Please find page 6 of the 2011 Eli Lilly annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
4
pay the dividend at least at its current level. It’s critically
important to management, our board of directors, and
shareholders—and we have no intention of cutting it.
Innovation for the long run
As we look to the future beyond YZ, Lilly remains fi rmly
committed to innovation. Our company is not shifting its
focus to generics or consumer products. And we’re not
pursuing a big merger. We’re committed to maintaining
our investment in R&D through the
current period of patent expirations,
and we’re keeping our focus on the
keys to Lilly’s long-term success:
• a pipeline that refl ects successful and
productive pursuit of innovation,
• an external environment that enables
medical innovation to fl ourish, and
• the transformation of our own com-
pany to ensure that we can continue
to deliver innovative medicines
valued by our customers.
Replenishing and advancing our
pipeline
The future growth—indeed, the very
survival—of our company depends
on a robust pipeline of innovative
medicines that meet the needs of pa-
tients. We ended 2011 with the highest
number of molecules we’ve ever had in
Phase III development, and through the year we sustained
a strong fl ow of new molecules into the pipeline. Overall,
since our 2010 annual report, we’ve moved 13 new assets
into Phase I, eight into Phase II, and four into Phase III,
and we’ve launched one new medicine, while terminating
the development of 12 molecules. (See page 9.)
With the addition of these molecules, we have 12 potential
new medicines in Phase III testing, surpassing our goal of
10 by the end of 2011. The large majority of the molecules
in our Phase III portfolio come with convincing Phase
II clinical data. And I’m pleased to note that 11 of the 12
assets come from our own labs, including ImClone.
New entries into Phase III since our 2010 annual report
include mGlu2/3 receptor agonist, now known as pomaglu-
metad methionil, for schizophrenia; the anti-IL-17 mono-
clonal antibody, now ixekizumab, for psoriasis; and two
potential treatments for diabetes—a novel basal insulin
analog and a new insulin glargine product.
Two Phase III trials for solanezumab, our monoclonal an-
tibody being studied as a potential treatment to slow the
progression of mild to moderate Alzheimer’s disease, are
scheduled to be completed in the second half of 2012. We
have additional molecules in our pipeline for Alzheimer’s,
including our oral BACE inhibitor, which will initiate a
Phase Ia/IIb study this year.
Another late-stage molecule, ramucirumab from our Im-
Clone unit, reached full enrollment in a Phase III breast
cancer trial in November 2011. This is one of six ongoing
Phase III trials with ramucirumab—as a single agent or in
combination with chemotherapy—in
fi ve different tumor types: liver,
gastric, colorectal, non-small cell lung,
and breast cancers.
Yet another molecule that we expect
to advance into Phase III testing in
2012 is our CETP inhibitor, evacetra-
pib. At the American Heart Associa-
tion meeting in November 2011, we
shared encouraging Phase II data
showing that evacetrapib, in combina-
tion with each of three commonly
used statins, increased HDL choles-
terol and also incrementally decreased
LDL cholesterol, both in a statistically
signifi cant manner. It’s important to
note that Phase III studies will still
take years to complete and this is a
very competitive area of research.
These molecules exemplify the progress we’ve made
in building a robust, high-quality, mid- to late-stage
pipeline, with an even mix of large and small molecules.
We believe our current pipeline includes many potential
opportunities to treat diseases with large unmet need, as
well as signifi cant commercial opportunity, and provides
the foundation for Lilly to return to growth after 2014.
This year, we’ll begin to generate and disseminate
important data that should help you better gauge our
growth potential.
Advocating for an environment for innovation
The second key to our long-term strategy is fostering
an external environment supportive of the pursuit of
innovation. We remain vigilant in advocating policies that
are essential to the continued viability of our innovation
model and essential for the discovery and development of
new medicines.
In 2011, we saw some positive developments, most no-
tably comprehensive patent reform in the United States
and important progress in the area of international trade,
including the U.S. trade agreement with South Korea.
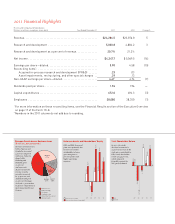
Key Contributors to 2011 Revenue Growth
($ in millions represent growth in revenue,
percent growth)
Seven products and a
product line—Cymbalta,
Humalog, Alimta, Effient,
Cialis, Humulin, Forteo,
and Animal Health—
generated $15.0 billion in
revenue during 2011, an
increase of $2.2 billion in
revenue over 2010. This
growth was driven
primarily by volume
increases.
Cymbalta
Humalog
Total Animal Health
Alimta
Effient
Cialis
Humulin
Forteo
$252.6 +11%
$287.2 +21%
$702.6 +20%
$313.4 +15%
$187.5 +163%
$176.2 +10%
$159.9 +15%
$119.7 +14%