Eli Lilly 2008 Annual Report Download - page 4
Download and view the complete annual report
Please find page 4 of the 2008 Eli Lilly annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
2
LETTER TO SHAREHOLDERS
To Our Shareholders
For Eli Lilly and Company, 2008 was a year of transition
and transformation.
Our solid fi nancial performance, driven by volume-
based sales growth, improved gross margins, and better
productivity, allowed us to make important investments
to advance our pipeline of promising molecules, to
resolve much of the uncertainty surrounding product liti-
gation, and to complete several strategic business develop-
ment transactions, including the acquisition of ImClone
Systems—the largest acquisition in Lilly history.
Transformation is not optional. The economic down-
turn only added to the challenges facing the pharmaceuti-
cal industry—including pressure on pricing and access,
a drought in research, and regulatory uncertainty. At the
same time, we have unprecedented opportunities to ad-
dress unmet patient needs. Lilly enters 2009 with more
molecules in clinical development than ever before—and
an unwavering commitment to deliver improved out-
comes for individual patients.
This has also been a year of transition. I succeeded
Sidney Taurel as CEO in April and as chairman on Janu-
ary 1, 2009. In my new responsibilities, I retain a pro-
found sense of optimism about Lilly’s future—grounded
in a realistic assessment of the challenges we face and the
diffi cult nature of the task ahead.
REVIEW OF 2008
Sales and fi nancial results
Throughout 2008, we advanced Lilly’s transformation
by executing on our operational and strategic priorities.
Reported sales grew 9 percent, driven primarily by a
5 percent increase in volume. For the fi rst time, we sur-
passed $20 billion in revenue, with eight products—and
our Elanco animal health business—exceeding $1 billion
in annual sales. According to data from IMS Health, Lilly
has moved into the top 10 companies in worldwide phar-
maceutical sales.
As a result of certain signifi cant charges, we reported
a net loss of $2.07 billion, or $1.89 per share, for 2008,
compared with 2007 net income of $2.95 billion and
earnings per share of $2.71. The company recorded total
charges of $4.73 billion related to the acquisition of
ImClone Systems, and $1.42 billion related to Zyprexa®
investigations by the United States Attorney for the East-
ern District of Pennsylvania (EDPA) and multiple states—
which I’ll discuss below. On a pro forma non-GAAP basis,
excluding signifi cant items totaling $5.91 per share, earn-
ings rose 14 percent to $4.02 per share.
Strong volume sales, coupled with discipline on
expenses and continued productivity gains, allowed us
to generate over $7 billion in operating cash fl ow. These
results give us the benefi t of a strong fi nancial position
just when we need it most—to make the necessary invest-
ments in our pipeline and in the company’s broader trans-
formation. We aim to sustain solid operating performance
as we prepare for the full impact of patent expirations
beginning in late 2011, a period we call “Years YZ.”
Commercial and regulatory overview
In 2008, we experienced three quarters of double-digit,
volume-driven sales growth that was broad-based across
many brands and regions. Unfortunately, in the fourth
quarter we saw a slowdown in total sales growth and vol-
ume growth. In addition, as the dollar strengthened late in
the year, exchange rates turned from a benefi t to a drag on
our sales line.
For the full year, products launched this decade—
Alimta®, Byetta®, Cialis®, Cymbalta®, Forteo®, Strattera®,
Symbyax®, Xigris®, and Yentreve™—collectively grew
22 percent on a reported basis, to $7.31 billion, and
accounted for 36 percent of total sales, compared with
32 percent of total sales in 2007. (For individual product
performance, please see page 15.)
In 2008, we set the stage for continued growth in our
marketed products with the approval and launch of new
indications and line extensions. These included: Alimta for
fi rst-line treatment of non-squamous non-small cell lung
cancer in the U.S. and Europe; Cymbalta for fi bromyalgia
in the U.S. and for generalized anxiety disorder in Europe;
Cialis for once-daily use in the U.S.; and the Humalog
KwikPen™ in the U.S., Japan, and select international mar-
kets. Zypadhera™—a long-acting formulation of Zyprexa—
received fi nal approval in Europe late last year, and we are
currently launching in the fi rst several markets.
In addition, we submitted, among others: Alimta for
the maintenance treatment of non-squamous non-small
cell lung cancer in the U.S. and Europe; Cialis for pulmo-
nary arterial hypertension in the U.S., Europe, and Japan;
and Byetta for monotherapy in the U.S.
As this report went to press, we received good
news in Europe on prasugrel, the antiplatelet agent we
0
$1,000
$2,000
$3,000
$4,000
$5,000
Zyprexa
Cymbalta
Humalog
Gemzar
Cialis
Alimta
Animal Health
Evista
Humulin
Forteo
Strattera
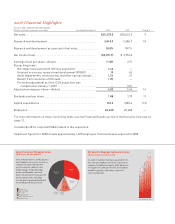
Eight Products Exceed $1 Billion
in Net Sales
($ millions)
Eight products and one product
line—Zyprexa, Cymbalta, Humalog,
Gemzar, Cialis, Alimta, Animal
Health, Evista, and Humulin—
exceeded $1 billion in 2008.
At $1.15 billion in sales, Alimta
reached “blockbuster” status in
its fifth year on the market.
$1,720
$1,736
$4,696
$2,697
$1,445
$1,155
$1,063
$1,076
$1,093
$779
$580