Eli Lilly 2012 Annual Report Download - page 5
Download and view the complete annual report
Please find page 5 of the 2012 Eli Lilly annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
3
Our Plan and 2012 Results
At our investor conference in December 2009, we
described how the entry of generic competition for
Zyprexa in key markets beginning in 2011 would usher
in a period when we lose patent protection for several of
our largest products.
We outlined several important nancial goals for this
so-called “YZ” period, 2012 to 2014. We said for every
year during YZ, we would achieve revenue of at least
$20 billion, net income of at least $3 billion, and operat-
ing cash ow of at least $4 billion.
We also stated that after this period we would resume
growth by launching a signicant portion of our Phase III
pipeline.
Achieving our nancial goals enables us to oper-
ate our business eectively, to continue to advance our
pipeline, and to provide returns to shareholders by
maintaining our dividend at least at its current level, and
by repurchasing shares.
In 2012, although our total revenue and earnings
declined due to the Zyprexa patent losses, we exceeded
our threshold performance goals through growth of
other products. Revenue was $22.6 billion, a decline of
7 percent from 2011. Excluding Zyprexa, the rest of our
worldwide revenue grew 6 percent for the year.
We rmly controlled costs while investing in R&D
to replenish and advance our pipeline. Our nancial
performance allowed us to repurchase Lilly shares while
maintaining the dividend. And for the second year in a
row, we generated total shareholder returns in the
mid-20 percent range. (See graph on page 1.)
Going forward, we’ll continue to focus on the three
strategic priorities that have guided our eorts thus far:
• Driving strong performance of our marketed brands
and key growth areas,
• Increasing productivity and reducing our cost
structure, and
• Advancing the pipeline.
Let me provide a more extensive review of Lilly’s
accomplishments in 2012 in each of these areas.
Driving Strong Performance
In 2012, eight of our products, plus our Elanco animal
health business, exceeded $1 billion in sales. Three key
products—Cymbalta®, Forteo®, and Eent®—achieved
double-digit growth, as did Elanco. And we continued to
pursue new indications and line extensions to sustain
growth across our portfolio.
We launched Amyvid™—a rst-of-its-kind diagnostic
for patients with cognitive impairment who are be-
ing evaluated for Alzheimer’s disease—in the U.S. We
received approval in Europe early this year, and we’re
working to gain approvals in Japan and select emerging
markets. Also in 2012, we gained the rst simultaneous
approval for a Lilly medicine with a companion diag-
nostic, when Erbitux® was approved in the U.S. for the
treatment of rst-line metastatic colorectal cancer along
with a diagnostic test that identies patients best suited
for the treatment.
In other approvals last year, the U.S. Food and Drug
Administration (FDA) and European Commission (EC)
both approved Jentadueto®—which combines Tradjenta®
and metformin in a single pill—and both also approved
Tradjenta as add-on therapy to insulin. The FDA ap-
proved Alimta® as a maintenance therapy following
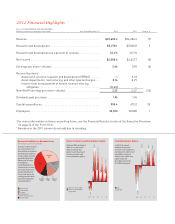
John C. Lechleiter, Ph.D.,
Chairman, President, and Chief
Executive Ofcer (center),
meets with Lilly scientists and
team leaders who have played
instrumental roles in the discovery
and development of solanezumab.
With John are (seated, left to
right) Richard Mohs, Ph.D.,
Early-Phase Clinical Leader and
Distinguished Research Fellow,
Neuroscience Research; Hong
Liu-Seifert, Ph.D., Research
Advisor, Global Statistical Sciences
and Advanced Analytics; Eric
Siemers, M.D., Senior Director—
Medical, Alzheimer’s Disease
Team; (standing, left to right)
Jirong Lu, Research Fellow,
Biotechnology Discovery Research;
Phyllis Barkman Ferrell, Global
Brand Development Leader,
Alzheimer’s Disease Team; Ronald
DeMattos, Ph.D., Research Fellow,
Neuroscience Discovery.
These talented individuals
epitomize Lilly expertise in
Alzheimer’s research and
development, and they represent
hundreds of Lilly colleagues
devoted to achieving progress
against this devastating disease.