Eli Lilly 2012 Annual Report Download - page 11
Download and view the complete annual report
Please find page 11 of the 2012 Eli Lilly annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
9
Over the 18 months of treatment,
the dierence between patients
treated with solanezumab and those
who received placebo increased at a
relatively constant rate over time. The
study results indicated that the safety
prole was reasonable for patients
with mild Alzheimer’s disease. Based
on discussions with experts in the
eld, we believe such an eect may
be considered to be clinically useful
by clinicians, caregivers, and patients.
Research continues . . .
Following discussions with global
regulators, we plan to conduct an
additional Phase III study of solanezu-
mab in patients with mild Alzheimer’s
disease, which we expect to initiate
no later than the third quarter of this
year. We are excited about the solan-
ezumab results, and we will continue
to analyze and review the data from
the two completed studies, including
the ongoing unblinded extensions of
both trials.
Solanezumab has been selected as
one of three experimental medicines
for evaluation in a worldwide clini-
cal study of early-onset Alzheimer’s
disease conducted by the Dominantly
Inherited Alzheimer’s Network
(DIAN) Trials Unit at Washington Uni-
versity School of Medicine. Our BACE
inhibitor is also being considered for
the study. The DIAN study will seek
to determine whether the potential
medicines are able to prevent the loss
of cognitive function in people with
inherited mutations that cause early-
onset Alzheimer’s disease.
In early 2013, researchers from
the Center for Alzheimer Research
and Treatment (CART) at Harvard’s
Brigham and Women’s Hospital,
along with the ADCS, announced
the selection of Lilly’s solanezumab
and Amyvid for use in another new
Alzheimer’s study. The A4 study,
as it is called, is designed to test a
molecule that targets amyloid-beta—
solanezumab—in older individuals
who have evidence of amyloid plaque
in their brains on an Amyvid scan,
but do not yet show symptoms of
memory impairment.
. . . as more pieces fall into place
Important new evidence on the key
role of amyloid-beta in Alzheimer’s
disease came last year from a paper
published in Nature. Researchers
in Iceland identied a rare genetic
mutation that slows the activity of
beta secretase, which is required to
produce amyloid-beta. Icelanders
with the benecial mutation are more
than ve times more likely than those
without it to reach age 85 without an
Alzheimer’s diagnosis.
In addition, our own results for
solanezumab support the amyloid
hypothesis, in that a molecule that
acts specically on amyloid-beta
demonstrated a slowing of cogni-
tive decline in patients with mild
Alzheimer’s disease.
Even as we remain committed to
research on amyloid-beta, we’re also
studying other potential pathways
that may play a role in Alzheimer’s
disease.
While Alzheimer’s is a scientic puz-
zle, it is also a human tragedy—one
that tears the mind of an individual
and the hearts of families, caregivers,
and friends. Its cruelty is magnied
because we seem defenseless against
it. Our work is all the more urgent
because we know that as populations
age, it is set to aect millions more.
Lilly is determined to make a dif-
ference. Our eorts on Alzheimer’s
disease exemplify our commitment
to—and the work of—pharmaceuti-
cal innovation. Dedicated experts
conduct painstaking research over
many years, learning from both
successes and failures—as, piece by
piece, answers to the world’s most
devastating diseases take shape. n
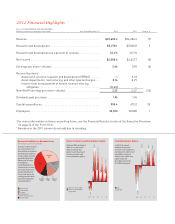
In the 25 years that Lilly has been pursuing answers to Alzheimer’s disease, AD has been
silently but insidiously progressing in the millions of patients worldwide who suffer from
Alzheimer’s disease today—with impacts on the brain occurring long before symptoms emerge.
9
Plaque deposition
in the brain begins
10-15 years before
the onset of dementia,
Joe’s memory is not
noticeably different
from others his age.
Brain metabolism
begins to slow
Joe has increasing
memory loss and prob-
lems understanding
language and express-
ing himself. He some-
times writes reminders
for himself for daily
activities. Joe’s doctor
describes his condi-
tion as “Mild Cognitive
Impairment.”
Onset of
dementia
Joe has persistent
memory loss and needs
help with daily living.
He sometimes becomes
lost and disoriented.
Joe’s doctor describes
his condition as
“probable Alzheimer’s
disease.”
Severe Alzheimer’s
disease
5 years after the onset
of dementia, Joe is
unable to recognize
his family or com-
municate with them.
He has problems with
swallowing, inconti-
nence, and occasional
delirium. He requires
round-the-clock care.
INSIDIOUS FOE, INEXORABLE DECLINE
AD progression in a patient suffering from dementia today*
*This illustration is not intended to be representative of every patient’s experience with AD.