Eli Lilly 2012 Annual Report Download
Download and view the complete annual report
Please find the complete 2012 Eli Lilly annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
PROGRESS AGAINST ALZHEIMER’S DISEASE
Eli lilly and Company
2012 annual REpoRt
notiCE of 2013 annual mEEting
pRoxy StatEmEnt
Table of contents
-
Page 1
P RO G R E S S A GA I N S T A L Z H E I M E R ' S D I S E A S E Eli lilly and Company 2012 annual REpoRt notiCE of 2013 annual mEEting pRoxy StatEmEnt -
Page 2
...the Board and Its Committees Directors and Corporate Governance Committee Matters Audit Committee Matters Compensation Committee Matters Compensation Discussion and Analysis Executive Compensation Summary Compensation Table Ownership of Company Stock Items of Business To Be Acted Upon at the Meeting... -
Page 3
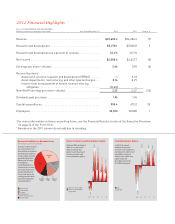
2012 Financial Highlights ELi LiLLy and Company and SubSidiariES (dollars in millions, except per-share data) year Ended december 31 2012 2011 Change % revenue...research and development ...research and development as a percent of revenue...net income ...Earnings per share-diluted...reconciling ... -
Page 4
... sales and earnings. At the same time, we began to see the first shoots of our innovation strategy emerge. One promising area of Lilly research-our work to combat Alzheimer's disease-is highlighted in this report. The pages following this letter provide a glimpse into the medical challenge and human... -
Page 5
... devastating disease. Achieving our financial goals enables us to operate our business effectively, to continue to advance our pipeline, and to provide returns to shareholders by maintaining our dividend at least at its current level, and by repurchasing shares. In 2012, although our total revenue... -
Page 6
... Contributors to 2012 Revenue costs. six months of market exclusivity for ($ in millions represent growth in revenue, percent growth) Following the YZ period, we Cymbalta in the U.S.-to December expect to return to levels of R&D 2013-after meeting the FDA's Four products and a product spending more... -
Page 7
... with our Lilly trial in psoriasis of our anti-IL-17 monoclonal antibody people. I have the good fortune to work alongside thousands ixekizumab. of talented and dedicated colleagues. I especially want to In January 2013, Lilly and Boehringer Ingelheim recognize and thank two members of our Executive... -
Page 8
... complex and devastating disease. Since embarking on Alzheimer's disease research 25 years ago, Eli Lilly and Company has brought together some of the world's leading Alzheimer's experts in an effort to discover and develop treatments that will offer new hope to patients and those who care for them... -
Page 9
... the most basic human aspects of personality, memory, and individuality. Jan Lundberg, Ph.D. Executive Vice President, Science and Technology, and President, Lilly Research Laboratories While there are still many theories about what causes Alzheimer's disease, the most productive line of *data... -
Page 10
...-beta agent to show a slowing of cognitive decline in patients with mild Alzheimer's disease. Independent analyses of the Phase III solanezumab data were conducted by the Alzheimer's Disease Cooperative Study (ADCS), an academic research consortium, and were found to be generally similar to Lilly... -
Page 11
... occasional delirium. He requires round-the-clock care. Following discussions with global regulators, we plan to conduct an additional Phase III study of solanezumab in patients with mild Alzheimer's disease, which we expect to initiate no later than the third quarter of this year. We are excited... -
Page 12
... insulin Glargine Product* diabetes Empagliflozin* diabetes diabetes p38 mapK inhibitor i cancer ron mab cancer mr antagonist chronic kidney disease CXCr4 peptide inhibitor cancer The Lilly pipeline currently includes 64 molecules in clinical development. Since our last annual report, 10 new... -
Page 13
... 31, 2012 Commission file number 001-06351 Eli Lilly and Company An Indiana corporation I.R.S. employer identification no. 35-0470950 Lilly Corporate Center, Indianapolis, Indiana 46285 (317) 276-2000 Securities registered pursuant to Section 12(b) of the Act: Title of Each Class Common Stock (no... -
Page 14
...new products; regulatory actions regarding currently marketed products; issues with product supply; regulatory changes or other developments; regulatory compliance problems or government investigations; our ability to protect and enforce patents and other intellectual property; changes in patent law... -
Page 15
... succeed to the drug manufacturing business founded in Indianapolis, Indiana, in 1876 by Colonel Eli Lilly. We discover, develop, manufacture, and market products in two business segments-human pharmaceutical products and animal health products. The mission of our human pharmaceutical business is to... -
Page 16
... of bacterial infections. Animal Health Products Our products for food animals include 4 Rumensin®, a cattle feed additive that improves feed efficiency and growth and also controls and prevents coccidiosis Tylan®, an antibiotic used to control certain diseases in cattle, swine, and poultry... -
Page 17
... about our major products. We supplement our employee sales force with contract sales organizations as appropriate to leverage our own resources and the strengths of our partners in various markets. We maintain special business groups to service wholesalers, pharmacy benefit managers, managed-care... -
Page 18
... other companies that operate animal health businesses. Important competitive factors include safety, effectiveness, and ease of use of our products; price and demonstrated cost-effectiveness; marketing effectiveness; and research and development of new products and processes. Most new products that... -
Page 19
... Food and Drug Administration (FDA). A single patent for a human pharmaceutical product may be eligible for patent term restoration, to make up for a portion of the time invested in clinical trials and the FDA review process. Patent term restoration is limited by a formula and cannot be calculated... -
Page 20
... law, a drug or biological product can receive "orphan" designation if it is intended to treat a disease or condition affecting fewer than 200,000 people in the U.S., or affecting more than 200,000 people but not reasonably expected to recover its development and marketing costs through U.S. sales... -
Page 21
... event that Lilly becomes bankrupt or insolvent. • Patent Challenges In the U.S., the Drug Price Competition and Patent Term Restoration Act of 1984, commonly known as the Hatch-Waxman Act, made a complex set of changes to both patent and new-drug-approval laws for human pharmaceuticals. Before... -
Page 22
... health product regulations address the administration of the product in or on the animal, and in the case of food animal products, the impact on humans who consume the food as well as the impact on the environment at the production site. The laws and regulations affecting the manufacture and sale... -
Page 23
...price controls, reference pricing, discounts and rebates, restrictions on physician prescription levels, restrictions on reimbursement, compulsory licenses, health economic assessments, and generic substitution. The 2010 enactment of the Patient Protection and Affordable Care Act and The Health Care... -
Page 24
.... We use the services of physicians, hospitals, medical schools, and other research organizations worldwide to conduct clinical trials to establish the safety and effectiveness of our human pharmaceutical products. We actively seek out investments in external research and technologies that hold the... -
Page 25
...and launch of a product, we expend considerable resources on post-marketing surveillance and clinical studies to collect and understand the benefits and potential risks of medicines as they are used as therapeutics. The following describes the new drug research and development process in more detail... -
Page 26
..., and packaging, take place at a number of sites throughout the world. In 2010, we sold our Tippecanoe Laboratories manufacturing site in West Lafayette, Indiana, to an affiliate of Evonik Industries AG, and entered into a nine-year supply and services agreement whereby Evonik manufactures final and... -
Page 27
...mayor of Indianapolis, Indiana from 2000 to 2007. From 2008 to 2009, he was managing director at Strategic Capital Partners, LLC, and distinguished visiting professor of public policy at Ball State University. Executive Vice President, Global Services (since January 2010) and Chief Financial Officer... -
Page 28
... in research and development and capital as well as other expenditures required to bring new drugs to the market. Intellectual property protection varies throughout the world and is subject to change over time. In the U.S., the Hatch-Waxman Act provides generic companies powerful incentives... -
Page 29
... of U.S. health care reform legislation is increasing these pricing pressures. In addition, many state legislative proposals would further negatively affect our pricing and reimbursement for, or access to, our products. Globally, public and private payers are increasingly restricting access to human... -
Page 30
...issues. We are now operating under a Corporate Integrity Agreement with the Office of Inspector General of the U.S. Department of Health and Human Services that requires us to maintain comprehensive compliance programs governing our research, manufacturing, and sales and marketing of pharmaceuticals... -
Page 31
... Comments None. Item 2. Properties Our principal domestic and international executive offices are located in Indianapolis. At December 31, 2012, we owned 13 production and distribution sites in the U.S. and Puerto Rico. Together with the corporate administrative offices, these facilities contain... -
Page 32
...defendant in product liability cases in the U.S. related to the diabetes medication Actos, which we co-promoted with Takeda in the U.S. from 1999 until September 2006. In addition, we have been named along with Takeda as a defendant in four purported product liability class actions in Canada related... -
Page 33
...set of defined corporate integrity obligations for five years. The agreement also provides for an independent third-party review organization to assess and report on the company's systems, processes, procedures, and practices related to compliance with health care laws. In December 2010, we received... -
Page 34
... for prices in the U.S. in violation of antitrust laws. The case sought restitution for alleged overpayments for human pharmaceuticals and an injunction against the allegedly violative conduct. In March 2011, the trial court granted summary judgment for us and the other defendants. In August 2012... -
Page 35
... 2012: Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs (in thousands) Approximate Dollar Value of Shares that May Yet Be Purchased Under the Plans or Programs (dollars in millions) Period Total Number of Shares Purchased (in thousands) Average Price Paid per Share... -
Page 36
Financial Results Worldwide total revenue decreased 7 percent to $22.60 billion in 2012, driven by steep sales declines for Zyprexa due to the loss of patent exclusivity in most major markets, partially offset by growth in certain other products. Net income and EPS decreased 6 percent to $4.09 ... -
Page 37
... growth factor receptor (EGFR) monoclonal antibody for the treatment of squamous non-small cell lung cancer (NSCLC) New insulin glargine product (Q3 2011)-a new insulin glargine product for the treatment of type 1 and type 2 diabetes (in collaboration with Boehringer Ingelheim) Novel basal insulin... -
Page 38
... and cost more than $1 billion. Failure can occur at any point in the process, including late in the process after substantial investment. As a result, most research programs will not generate financial returns. New product candidates that appear promising in development may fail to reach the market... -
Page 39
.... Operating Results-2012 Revenue Our worldwide revenue for 2012 decreased 7 percent, to $22.60 billion, driven by the loss of patent exclusivity for Zyprexa in most major markets, partially offset by growth in Cymbalta, Forteo, Effient, Alimta, and our animal health portfolio. Worldwide sales volume... -
Page 40
... includes revenue in Puerto Rico. Collaboration and other revenue consists primarily of royalties for Erbitux and revenue associated with exenatide in the United States. Sales of Cymbalta, a product for the treatment of major depressive disorder, diabetic peripheral neuropathic pain, generalized... -
Page 41
... human insulin for the treatment of diabetes, increased 1 percent in the U.S., driven by higher prices, largely offset by decreased demand. U.S. sales of Humulin were negatively affected by the product's removal from a large formulary in 2012, as well as the continued decline in the market for human... -
Page 42
... with the diabetes collaboration with Boehringer Ingelheim, as well as a benefit from the resolution in 2011 of the IRS audits of tax years 2005-2007, along with certain matters related to 2008-2009. See Note 13 to the consolidated financial statements for additional information. Operating Results... -
Page 43
...Percent Change from 2010 Zyprexa ...$ 2,165.3 Cymbalta ...3,173.4 Alimta ...994.6 Humalog ...1,398.9 Cialis ...704.5 Humulin...588.1 Evista ...707.5 Forteo ...453.1 Strattera ...392.2 Gemzar ...70.6 Other pharmaceutical products ...879.4 Animal health products ...896.8 Total net product sales ...12... -
Page 44
... pharmaceutical manufacturers' fee associated with U.S. health care reform. Investment in research and development increased 3 percent, to $5.02 billion, due primarily to increased late-stage clinical trial costs, including costs related to the diabetes collaboration with Boehringer Ingelheim. We... -
Page 45
... business, our operations are exposed to fluctuations in interest rates and currency values. These fluctuations can vary the costs of financing, investing, and operating. We address a portion of these risks through a controlled program of risk management that includes the use of derivative financial... -
Page 46
...financial condition, revenues or expenses, results of operations, liquidity, capital expenditures, or capital resources. We acquire and collaborate on assets still in development and enter into research and development arrangements with third parties that often require milestone and royalty payments... -
Page 47
... of operations, financial position, or liquidity for the periods presented in this report. Our most critical accounting estimates have been discussed with our audit committee and are described below. Revenue Recognition and Sales Return, Rebate, and Discount Accruals We recognize revenue from sales... -
Page 48
..., long-term care, hospital, patient assistance programs, and various other programs. We base these accruals primarily upon our historical rebate and discount payments made to our customer segment groups and the provisions of current rebate and discount contracts. The largest of our sales rebate... -
Page 49
... reported. In addition to the analysis below, see Note 14 to the consolidated financial statements for additional information regarding our retirement benefits. Annually, we evaluate the discount rate and the expected return on plan assets in our defined benefit pension and retiree health benefit... -
Page 50
... 2012 discount rate for the U.S. defined benefit pension and retiree health benefit plans (U.S. plans) were to be changed by a quarter percentage point, income before income taxes would change by $35.2 million. If the 2012 expected return on plan assets for U.S. plans were to be changed by a quarter... -
Page 51
...of products including Humalog, Humulin, Cialis, Strattera, Forteo, Alimta, Cymbalta outside the U.S., Effient, Tradjenta, and Axiron, as well as animal health products. In addition, significant revenue growth is expected in Japan and the emerging markets, particularly China. We anticipate that gross... -
Page 52
Item 8. Financial Statements and Supplementary Data Consolidated Statements of Operations ELI LILLY AND COMPANY AND SUBSIDIARIES (Dollars in millions, except per-share data) Year Ended December 31 2012 2011 2010 Revenue ...Cost of sales ...Research and development ...Marketing, selling, and ... -
Page 53
... ELI LILLY AND COMPANY AND SUBSIDIARIES (Dollars in millions) Year Ended December 31 2012 2011 2010 Net income ...Other comprehensive income (loss) Foreign currency translation gains (losses) ...Net unrealized gains (losses) on securities ...Defined benefit pension and retiree health benefit plans... -
Page 54
... ...Property and equipment, net ...Total assets ...Liabilities and Shareholders' Equity Current Liabilities Short-term borrowings and current maturities of long-term debt (Note 8) ...Accounts payable...Employee compensation...Sales rebates and discounts...Dividends payable ...Income taxes payable... -
Page 55
... ...Change in deferred income taxes ...Stock-based compensation expense ...Impairment charges, indefinite lived intangibles ...Acquired in-process research and development, net of tax...Income related to prepayment of revenue-sharing obligation (Note 4)...Other operating activities, net ...Changes... -
Page 56
... We operate in two business segments-human pharmaceutical products and animal health. Our business segments are distinguished by the ultimate end user of the product-humans or animals. Performance is evaluated based on profit or loss from operations before income taxes. The accounting policies of... -
Page 57
...reporting presented to the chief operating decision maker, certain costs are fully allocated to our human pharmaceutical products segment and therefore are not reflected in the animal health segment's profit. Such items include costs associated with treasury-related financing, global service centers... -
Page 58
Selected Quarterly Data (unaudited) ELI LILLY AND COMPANY AND SUBSIDIARIES (Dollars in millions, except per-share data) 2012 Fourth Third Second First Revenue ...Cost of sales ...Operating expenses ...Acquired in-process research and development...Asset impairments, restructuring, and other special... -
Page 59
...) ELI LILLY AND COMPANY AND SUBSIDIARIES (Dollars in millions, except revenue per employee and per-share data) 2012 2011 2010 2009 2008 Operations Revenue ...$ 22,603.4 $ 24,286.5 $ 23,076.0 $ 21,836.0 $ 20,371.9 Cost of sales ...4,796.5 5,067.9 4,366.2 4,247.0 4,376.7 Research and development... -
Page 60
... Lilly stock, the S&P 500 Stock Index, and the peer group's common stock. The graph measures total shareholder return, which takes into account both stock price and dividends. It assumes that dividends paid by a company are reinvested in that company's stock. Value of $100 Invested on Last Business... -
Page 61
...Investment securities with maturity dates of less than one year from the date of the balance sheet are classified as short-term. Available-for-sale securities are carried at fair value with the unrealized gains and losses, net of tax, reported in other comprehensive income (loss). The credit portion... -
Page 62
... flow hedge. Management reviews the correlation and effectiveness of our derivatives on a quarterly basis. For derivative contracts that are designated and qualify as fair value hedges, the derivative instrument is marked to market with gains and losses recognized currently in income to offset the... -
Page 63
... is stated on the basis of cost. Provisions for depreciation of buildings and equipment are computed generally by the straight-line method at rates based on their estimated useful lives (12 to 50 years for buildings and 3 to 18 years for equipment). We review the carrying value of long-lived assets... -
Page 64
... approval for marketing or launch of the product), we amortize the payment to income as we perform under the terms of the arrangement. See Note 4 for specific agreement details. Royalty revenue from licensees, which is based on third-party sales of licensed products and technology, is recorded... -
Page 65
... generally equals the vesting period. Under our policy, all stock-based awards are approved prior to the date of grant. The compensation committee of the board of directors approves the value of the award and date of grant. Stock-based compensation that is awarded as part of our annual equity grant... -
Page 66
... approval in the U.S. in 2012 and European Union in 2013, and is available to a limited number of imaging centers. Alnara On July 20, 2010, we acquired all of the outstanding stock of Alnara, a privately-held company developing protein therapeutics for the treatment of metabolic diseases, for total... -
Page 67
... December 31, 2013. We are responsible for certain development costs related to certain clinical trials outside the U.S. that we were conducting as of the date of the termination agreement as well as commercialization costs outside the U.S. until the commercial operations are transferred to Amylin... -
Page 68
Under the terms of our prior arrangement, we reported as collaboration and other revenue our 50 percent share of gross margin on Amylin's net product sales in the United States. We reported as net product sales 100 percent of sales outside the U.S. and our sales of Byetta pen delivery devices to ... -
Page 69
... in the agreement were our new insulin glargine product and our novel basal insulin analog, both of which began Phase III clinical testing in the second half of 2011; and an option granted to Boehringer Ingelheim to co-develop and co-commercialize our anti-TGF-beta monoclonal antibody, which is... -
Page 70
... market, and promote Cymbalta (duloxetine) outside the U.S. and Japan. Pursuant to the terms of the agreement, we generally shared equally in development, marketing, and selling expenses, and paid Boehringer Ingelheim a commission on sales in the co-promotion territories. We manufactured the product... -
Page 71
... of an asset impairment associated with the decision to stop development of a delivery device platform, and $20.0 million resulting from a change in our estimates of returned product related to the withdrawal of Xigris from the market during the fourth quarter of 2011. For the year ended December 31... -
Page 72
... Effect of Risk Management Instruments on the Statement of Operations The following effects of risk-management instruments were recognized in other-net, (income) expense: 2012 2011 2010 Fair value hedges Effect from hedged fixed-rate debt...$ Effect from interest rate contracts...Cash flow hedges... -
Page 73
... Prices in Active Markets for Identical Assets (Level 1) Significant Other Observable Inputs (Level 2) Significant Unobservable Inputs (Level 3) Description Carrying Amount Amortized Cost Fair Value December 31, 2012 Cash and cash equivalents ...$ 4,018.8 Short-term investments U.S. government... -
Page 74
...that was paid for Amyvid in the second quarter of 2012. We determine fair values based on a market approach using quoted market values, significant other observable inputs for identical or comparable assets or liabilities, or discounted cash flow analyses. The fair value of equity method investments... -
Page 75
... December 31, 2012, 2011, or 2010. IPR&D consists of the acquisition date fair value of products under development acquired in business combinations that have not yet achieved regulatory approval for marketing adjusted for subsequent impairments. As discussed in Note 1, we use the "income method" to... -
Page 76
... for marketing in a significant global jurisdiction (U.S., Europe, and Japan) and capitalized milestone payments. Other intangibles consist primarily of the amortized cost of licensed platform technologies that have alternative future uses in research and development, manufacturing technologies, and... -
Page 77
... statements of cash flows. At December 31, 2012, additional stock-based compensation awards may be granted under the 2002 Lilly Stock Plan for not more than 92.0 million shares. Performance Award Program PAs are granted to officers and management and are payable in shares of our common stock... -
Page 78
...over the weighted-average remaining requisite service period of 20 months. Restricted Stock Units RSUs are granted to certain employees and are payable in shares of our common stock. RSU shares are accounted for at fair value based upon the closing stock price on the date of grant. The corresponding... -
Page 79
... officers, management, and board members at exercise prices equal to the fair market value of our stock price at the date of grant. Options fully vested 3 years from the grant date and have a term of 10 years. Stock option activity during the year ended December 31, 2012 is summarized below: Shares... -
Page 80
...source of funds to assist us in meeting our obligations under various employee benefit plans. The cost basis of the shares held in the trust was $3.01 billion and $3.01 billion at December 31, 2012 and 2011, respectively, and is shown as a reduction in shareholders' equity. Any dividend transactions... -
Page 81
...stock options ...Diluted earnings per share ...$ 3.66 Note 13: Income Taxes Following is the composition of income tax expense: 2012 $ 4,347.7 1,113,923 3.90 $ 5,069.5 1,105,788 4.58 $ $ 1,113,967 $ 3.90 1,105,813 $ 4.58 2011 2010 Current Federal ...$ Foreign ...State ...Total current tax... -
Page 82
... 31 are as follows: 2012 2011 Deferred tax assets Compensation and benefits ...$ Tax credit carryforwards and carrybacks...Tax loss carryforwards and carrybacks ...Asset purchases ...Sale of intangibles...Debt ...Intercompany profit in inventories...Product return reserves...Contingencies ...Other... -
Page 83
... the benefit in 2012 and 2011 on international operations reported in the effective tax rate reconciliation above includes the foreign tax credit related to the excise tax. The U.S. health care legislation (both the primary Patient Protection and Affordable Care Act and the Health Care and Education... -
Page 84
... 14: Retirement Benefits We use a measurement date of December 31 to develop the change in benefit obligation, change in plan assets, funded status, and amounts recognized in the consolidated balance sheets at December 31 for our defined benefit pension and retiree health benefit plans, which were... -
Page 85
... prior service benefit related to our retiree health benefit plans. We do not expect any plan assets to be returned to us in 2013. The following represents our weighted-average assumptions as of December 31: Defined Benefit Pension Plans 2012 2011 2010 Retiree Health Benefit Plans 2012 2011 2010... -
Page 86
Net pension and retiree health benefit expense included the following components: Defined Benefit Pension Plans 2011 Retiree Health Benefit Plans 2011 2012 2010 2012 2010 Components of net periodic benefit cost Service cost ...$ 253.1 $ 236.3 $ 219.2 $ 63.3 $ 72.4 $ 56.5 Interest cost...455.1 ... -
Page 87
... to meet their obligations. The gross values of these derivative receivables and payables are not material to the global asset portfolio, and their values are reflected within the tables below. The defined benefit pension and retiree health benefit plan allocation for the U.S. and Puerto Rico... -
Page 88
... Class Total Defined Benefit Pension Plans Public equity securities U.S...International ...Fixed income Developed markets ...Emerging markets...Private alternative investments Hedge funds...Equity-like funds ...Real estate ...Other ...Total ...Retiree Health Benefit Plans Public equity securities... -
Page 89
...: Fixed Income: Developed Markets Hedge Funds Equity-like Funds Real Estate Total Defined Benefit Pension Plans Beginning balance at January 1, 2012...$ Actual return on plan assets, including changes in foreign exchange rates: Relating to assets still held at the reporting date ...Relating... -
Page 90
... Class Total Defined Benefit Pension Plans Public equity securities U.S...International...Fixed income Developed markets ...Emerging markets...Private alternative investments Hedge funds ...Equity-like funds ...Real estate ...Other ...Total ...Retiree Health Benefit Plans Public equity securities... -
Page 91
... of additional discretionary funding in the aggregate during the year ended December 31, 2013 to several of our global defined benefit pension and post-retirement health benefit plans. Note 15: Contingencies We are a party to various legal actions and government investigations. The most significant... -
Page 92
...on our future consolidated results of operations, liquidity, and financial position. We expect a loss of exclusivity for Alimta would result in a rapid and severe decline in future revenues in the relevant market. Byetta Product Liability Litigation We have been named as a defendant in approximately... -
Page 93
... was an expense of $30.8 million in 2012, a benefit of $64.4 million in 2011, and an expense of $27.3 million in 2010. The tax effect related to our defined benefit pension and retiree health benefit plans (Note 14) was an expense of $34.4 million in 2012, a benefit of $383.8 million in 2011, and an... -
Page 94
... Management of Eli Lilly and Company and subsidiaries is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934. We have global financial policies that govern critical areas... -
Page 95
... is to evaluate whether internal control over financial reporting was designed and operating effectively. John C. Lechleiter, Ph.D. Chairman, President, and Chief Executive Officer February 21, 2013 Derica W. Rice Executive Vice President, Global Services and Chief Financial Officer 83 -
Page 96
... of the Public Company Accounting Oversight Board (United States), Eli Lilly and Company and subsidiaries' internal control over financial reporting as of December 31, 2012, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations... -
Page 97
... with the standards of the Public Company Accounting Oversight Board (United States), the 2012 consolidated financial statements of Eli Lilly and Company and subsidiaries and our report dated February 21, 2013 expressed an unqualified opinion thereon. Indianapolis, Indiana February 21, 2013 85 -
Page 98
... SEC (such as this Form 10-K) is recorded, processed, summarized, and reported on a timely basis. Our management, with the participation of John C. Lechleiter, Ph.D., chairman, president, and chief executive officer, and Derica W. Rice, executive vice president, global services and chief financial... -
Page 99
... business conduct applicable to all employees worldwide and to our Board of Directors; and Code of Ethical Conduct for Lilly Financial Management, a supplemental code for our chief executive officer and all members of financial management that focuses on accounting, financial reporting, internal... -
Page 100
...our equity compensation plans is found in the Proxy Statement under "Item 4, Reapproval of Material Terms of Performance Goals for 2002 Lilly Stock Plan." That information is incorporated in this report by reference. Item 13. Certain Relationships and Related Transactions, and Director Independence... -
Page 101
... Value Award under the 2002 Lilly Stock Plan2 Form of Restricted Stock Unit under the 2002 Lilly Stock Plan2 The Lilly Deferred Compensation Plan, as amended2 The Lilly Directors' Deferral Plan, as amended2 The Eli Lilly and Company Bonus Plan, as amended2 The Eli Lilly and Company Executive Officer... -
Page 102
... Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized. Eli Lilly and Company By /s/ John C. Lechleiter John C. Lechleiter, Ph.D., Chairman of the Board, President, and Chief Executive Officer February 21, 2013 90 -
Page 103
...Chairman of the Board, President, and Chief Executive Officer, and a Director (principal executive officer) Executive Vice President, Global Services and Chief Financial Officer (principal financial officer) Vice President, Finance and Chief Accounting Officer (principal accounting officer) Director... -
Page 104
... Used In This Report Trademarks or service marks owned by Eli Lilly and Company or its subsidiaries or affiliates, when first used in this report, appear with an initial capital and are followed by the symbol ® or ™, as applicable. In subsequent uses of the marks in the report, the symbols are... -
Page 105
... Annual Meeting of Shareholders May 6, 2013 The annual meeting of shareholders of Eli Lilly and Company will be held at the Lilly Center Auditorium, Lilly Corporate Center, Indianapolis, Indiana, 46285 on Monday, May 6, 2013, at 11:00 a.m. EDT for the following purposes: • to elect five directors... -
Page 106
..., May 6, 2013 at: The Lilly Center Auditorium Lilly Corporate Center Indianapolis, Indiana 46285 The board of directors of Eli Lilly and Company, "we," "Lilly," or "the company," is soliciting proxies to be voted at the annual meeting and at any adjournment of the annual meeting. The record date for... -
Page 107
... can be found under "Director Compensation" below. Contacting the board of directors You may send written communications to one or more members of the board, addressed to: Board of Directors Eli Lilly and Company c/o Corporate Secretary Lilly Corporate Center Indianapolis, Indiana 46285 All such... -
Page 108
...past 10 years. Class of 2013 The following five directors' terms will expire at this year's annual meeting. See "Item 1. Election of Directors" below for more information. Ralph Alvarez, Age 57, Director since 2009 Executive Chairman, Skylark Co., Ltd. Mr. Alvarez is executive chairman of Skylark Co... -
Page 109
..., global operations, international business, and strategic planning. His international experience includes a special focus on emerging markets. Board committees: finance; public policy and compliance; science and technology Sir Winfried Bischoff, Age 71, Director since 2000 Chairman, Lloyds Banking... -
Page 110
...of leadership in operations and strategy. He is an audit committee financial expert as a result of his experience as CEO and CFO of Ball. He also has extensive corporate governance experience through his service on other public company boards. Board committees: audit; compensation Kimberly-Clark in... -
Page 111
... financial industry, including banking and financial services. Through her for-profit and her public-private partnership work, she has significant experience in international economics and finance. Ms. Horn has extensive corporate governance experience through service on other public company boards... -
Page 112
... of pharmaceutical research and development, sales and marketing, strategy, and operations. He also has significant corporate governance experience through service on other public company boards. Board committees: none Class of 2015 The following four directors will continue in office until 2015... -
Page 113
...the market price of the stock on the date dividends are paid. Actual shares are issued or transferred after the director ends his or her service on the board. • Deferred Compensation Account. Funds in this account earn interest each year at a rate of 120 percent of the applicable federal long-term... -
Page 114
... Exercise Price $65.48 $65.48 $65.48 $65.48 $65.48 $65.48 2 This column consists of amounts donated by the Eli Lilly and Company Foundation, Inc. under its matching gift program, which is generally available to U.S. employees as well as the outside directors. Under this program, the foundation... -
Page 115
...compensation committee of that company's board. • A director who is currently employed by, who is a 10 percent shareholder of, or whose immediate family member is currently employed as an executive officer of a company that makes payments to or receives payments from Lilly for property or services... -
Page 116
Members of board committees must meet all applicable independence tests of the NYSE, Securities and Exchange Commission (SEC), and Internal Revenue Service (IRS). The directors and corporate governance committee determined that all 13 nonemployee directors listed below are independent, and that the ... -
Page 117
... of Lilly stock. Directors are required to hold Lilly stock, directly or through company plans, valued at not less than five times their annual cash retainer; new directors are allowed five years to reach this ownership level. IV. Key Board Responsibilities Selection of Chairman and Chief Executive... -
Page 118
... The enterprise risk management program as a whole is reviewed annually at a joint meeting of the audit and public policy and compliance committees, and enterprise risks are also addressed at the annual board strategy session. Additional review or reporting on enterprise risks is conducted as needed... -
Page 119
... relevant committee will review the transaction annually to determine whether it continues to be in the company's best interests. The directors and corporate governance committee has approved the following related-party transactions. Dr. John Bamforth, senior director, chief marketing officer, Lilly... -
Page 120
..., technologies, and trends in pharmaceutical research and development; • reviews the progress of the company's new product pipeline; and • oversees matters of scientific and medical integrity and risk management. Membership and Meetings of the Board and Its Committees In 2012, each director... -
Page 121
... or retired chief executive officers and senior executives, particularly those with experience in operations, finance, accounting, banking, marketing, and sales; • international business; • medicine and science; • government and public policy; and • health care system (public or private... -
Page 122
..., as defined in the rules of the SEC. Audit Committee Report The audit committee reviews the company's financial reporting process on behalf of the board. Management has the primary responsibility for the financial statements and the reporting process, including the systems of internal controls and... -
Page 123
... testing under Section 404 of the Sarbanes-Oxley Act. The committee periodically meets with the internal and independent auditors, with and without management present, and in private sessions with members of senior management (such as the chief financial officer and the chief accounting officer... -
Page 124
...'s global compensation philosophy and policies, as well as establishes the compensation of executive officers. The committee also acts as the oversight committee with respect to the company's deferred compensation plans, management stock plans, and other management incentive compensation programs... -
Page 125
...hedging of company shares • negative compensation consequences for serious compliance violations and compensation recovery policy in place for executives • meaningful share ownership requirements for all members of senior management. The committee concluded the company's compensation programs do... -
Page 126
... and Analysis Summary Executive compensation for 2012 aligned well with the objectives of our compensation philosophy and with our performance, driven by these factors: • The company exceeded internal corporate goals for revenue and earnings per share (EPS) as well as pipeline progress, as... -
Page 127
... (between short- and long-term performance, internal and external metrics, cash and stock compensation, fixed and variable pay), and encourage employee retention and engagement. The Compensation Committee's Processes and Analyses Linking Business Strategy and Compensation Program Design At Lilly, we... -
Page 128
... group in 2010. The peer companies are direct competitors for our products, operate in a similar business model, and employ people with the unique skills required to operate an established biopharmaceutical company. The committee also considers market cap and revenue as measures of size. With the... -
Page 129
... markets. • Continued to advance the product pipeline, with 11 molecules in late-stage development. The directors also noted Dr. Lechleiter's strong leadership in establishing and executing the company's strategy to weather the period of patent expirations through 2014 and return to long-term... -
Page 130
... SVAs. Executives also received the company employee benefits package. This total compensation program balances the mix of cash and equity compensation, the mix of current and longer-term compensation, the mix of internally and externally focused goals, and the security of foundational benefits in... -
Page 131
...% • Company performance measures. A bonus program's goals should be challenging, yet achievable, in order to motivate and retain employees. Since 2011, performance goals under our bonus plan are tied directly to our internal annual operating goals. The committee established 2012 corporate goals... -
Page 132
... the 2002 Lilly Stock Plan: PAs and SVAs. These incentives are designed to focus company leaders on multi-year operational performance as well as long-term shareholder value. In 2012, the company granted equity incentives to approximately 15 percent of our employee population. For executive officers... -
Page 133
... in the form of equity. The committee determined that for members of senior management, a 50/50 split between PAs and SVAs appropriately balances the company financial Equity Compensation: • Performance metrics of growth in non-GAAP EPS and share price are objective and align with shareholder... -
Page 134
... of the closing prices of company stock for all trading days in November and December 2014. The 2012-2014 SVA will be paid out to executive officers according to the grid below in early 2015: 2012-2014 SVA Ending Stock Price Compounded Annual Share Price Growth Rate (excluding dividends) Percent of... -
Page 135
... Xigris® withdrawal) Eliminate impact of the early payment of Amylin financial obligation Non-GAAP EPS Xigris withdrawal adjustment EPS impact of the timing of pricing actions Pro-rata portion of Amylin net income 2012 R&D Tax Credit Non-GAAP EPS-adjusted *Numbers may not add due to rounding $22... -
Page 136
... revenue-sharing obligation; (ii) Added back the planned income from exenatide for the portion of the year after the early payment; • For 2010 and 2011: Eliminated one-time accounting charges for acquired in-process research and development; • For 2010: Eliminated the impact of U.S. health care... -
Page 137
...Post-Employment Benefits The company offers core employee benefits coverage to: • provide our global workforce with a reasonable level of financial support in the event of illness, injury, and retirement • enhance productivity and job satisfaction through programs that focus on work/life balance... -
Page 138
... be paid out on a pro-rated basis for time worked up to the change in control based on the merger price for company stock. • Covered terminations. Employees are eligible for payments if, within two years of the change in control, their employment is terminated (i) without cause by the company or... -
Page 139
... diversity of meaningful financial metrics (growth in stock price measured over three years, revenue, EPS [measured over one and two years], and pipeline progress), providing a balance between short- and long-term performance. The committee reviews incentive programs each year against the objectives... -
Page 140
...'s management stock plans, and other management incentive, benefit, and perquisite programs. Management has the primary responsibility for the company's financial statements and reporting process, including the disclosure of executive compensation. With this in mind, the compensation committee has... -
Page 141
..., Global Services and Chief Financial Officer Jan M. Lundberg, Ph.D. Executive Vice President, Science and Technology and President, Lilly Research Laboratories Robert A. Armitage Senior Vice President and General Counsel Enrique A. Conterno Senior Vice President and President, Lilly Diabetes... -
Page 142
...to such relocation-related tax reimbursements, which were made pursuant to our relocation policy that applies to any employee asked by the company to relocate. Relocation expenses reimbursed under the company relocation policy. We have no employment agreements with our named executive officers. 38 -
Page 143
... SVA, a participant must remain employed with the company through the end of the relevant performance period (except in the case of death, disability, or retirement). In addition, an employee who was an executive officer at the time of the 2012-2013 PA grant will receive payment in RSUs according to... -
Page 144
... Price ($) Option Expiration Date Award 2012-2014 SVA 2011-2013 SVA 2012-2013 PA 2011-2012 PA 2010-2011 PA Number of Shares or Units of Stock That Have Not Vested (#) Market Value of Shares or Units of Stock That Have Not Vested ($) Equity Incentive Plan Awards: Number of Unearned Shares, Units... -
Page 145
.... See the footnotes to "Summary Compensation Table" for information about company contributions for the named executive officers. • The retirement plan, a tax-qualified defined benefit plan that provides monthly benefits to retirees. See the "Pension Benefits in 2012" table below for additional... -
Page 146
... values. The benefits are not payable as a lump sum; they are generally paid as a monthly annuity for the life of the retiree and, if elected, any qualifying survivor. The annual benefit under the retirement plan is calculated using years of service and the average of the annual earnings (salary... -
Page 147
... did not change the timing or amount of his unreduced benefits (shown in the "Pension Benefits in 2012" table). A grant of additional years of service credit to any employee must be approved by the compensation committee of the board of directors. Nonqualified Deferred Compensation in 2012 Executive... -
Page 148
... payments and benefits under the company's compensation and benefit plans and arrangements to which the named executive officers would be entitled upon termination of employment. Except for certain terminations following a change in control of the company, as described below, there are no agreements... -
Page 149
... plan. Those amounts are shown in the "Nonqualified Deferred Compensation in 2012" table. Death and Disability. A termination of employment due to death or disability does not entitle named executive officers to any payments or benefits that are not available to salaried employees generally... -
Page 150
...payment pursuant to the change-in-control plan amounts to the benefit of two times the employee's 2012 annual base salary plus two times the employee's bonus target for 2012 under the bonus plan. • Continuation of medical and welfare benefits. This amount represents the present value of the change... -
Page 151
... includes the number of stock units credited to the directors' accounts in the Lilly Directors' Deferral Plan. 5 Mr. Armitage retired on December 31, 2012. 6 The shares shown for Dr. Lechleiter include 40,396 shares that are owned by a family foundation for which he is a director. Dr. Lechleiter... -
Page 152
... of the company's common stock, as of December 31, 2012, are the shareholders listed below: Name and Address Lilly Endowment, Inc. (the Endowment) 2801 North Meridian Street Indianapolis, Indiana 46208 BlackRock, Inc. 40 East 52nd Street New York, New York 10022 Number of Shares Beneficially Owned... -
Page 153
... for the 2002 Lilly Stock Plan Section 162(m) of the Internal Revenue Code limits the amount of compensation expense that the company can deduct for income tax purposes. In general, a public corporation cannot deduct compensation in excess of $1 million paid to any of the named executive officers in... -
Page 154
... goals specified in the 2002 Lilly Stock Plan. The material terms of those performance goals are: • earnings per share • net income • divisional income • corporate or divisional revenue • EVA® (after-tax operating profit less the annual total cost of capital) • Market Value Added (the... -
Page 155
... price below fair market value of Lilly stock on the date of grant, (iii) increase the number of shares authorized for issuance or transfer, or (iv) increase any of the maximum limits established for stock options and PAs. The committee may provide in the grant agreement, or by subsequent action... -
Page 156
... for the 2002 Lilly Stock Plan. Abstentions will not be counted either for or against these proposals. Quorum A majority of the outstanding shares, present or represented by proxy, constitutes a quorum for the annual meeting. As of the record date,1,129,678,645 shares of company common stock were... -
Page 157
...a small number of shares from a prior stock ownership plan, which can be voted only on the directions of the participants to whose accounts the shares are credited). All participants are named fiduciaries under the terms of the 401(k) plan and under the Employee Retirement Income Security Act (ERISA... -
Page 158
... November 25, 2013. Proposals should be addressed to the company's corporate secretary, Lilly Corporate Center, Indianapolis, Indiana 46285. In addition, the company's bylaws provide that any shareholder wishing to propose any other business at the annual meeting must give the company written notice... -
Page 159
....D. Senior Vice President, Product and Clinical: Design, Development, and Delivery Myles O'Neill Senior Vice President, Global Parenteral Drug Product and Delivery Devices Manufacturing Derica W. Rice Executive Vice President, Global Services, and Chief Financial Officer David A. Ricks Senior Vice... -
Page 160
...and 10-Q reports. In addition, the company's chief executive officer has filed with the New York Stock Exchange a certification to the effect that, to the best of his knowledge, the company is in compliance with all corporate governance listing standards of the Exchange. Transfer agent and registrar... -
Page 161
-
Page 162
... of this page with you to the meeting. detach here detach here Eli Lilly and Company annual meeting of Shareholders may 6, 2013 Complimentary Parking Lilly Corporate Center Please place this identifier on the dashboard of your car as you enter Lilly Corporate Center so it can be clearly seen by... -
Page 163
...toll-free 1.855.LLY.TRUE (1.855.559.8783) Partnership for Prescription Assistance (program sponsored by America's pharmaceutical research companies): www.pparx.org For perspectives on health care innovation LillyPAD, an official blog of Eli Lilly and Company: lillypad.lilly.com © 2013 Eli Lilly and... -
Page 164