Amgen 2009 Annual Report Download
Download and view the complete annual report
Please find the complete 2009 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Amgen 2009 Annual Report
and Financial Summary
Table of contents
-
Page 1
Amgen 2009 Annual Report and Financial Summary -
Page 2
...world ï¬ght cancer, kidney disease, rheumatoid arthritis, and other serious illnesses. With a number of novel potential new medicines in our pipeline, the next decade will mark a promising new chapter for Amgen- and for patients. We are conducting medical research and clinical trials in our current... -
Page 3
... 2009 Annual Report â- 1 Letter to Stockholders Dear Stockholders, In 2009, Amgen delivered vital medicines to patients as we weathered the most challenging economic environment in our 30-year history. We managed our business with ï¬scal discipline, generated more than $6 billion in operating... -
Page 4
... our access and patient assistance services under an â„¢ integrated program called Amgen Assist. important step on the way to approval. We expect Proliaâ„¢ approval this year in the United States, Europe, and other regions. In the United States, we have a world-class sales force hired and trained... -
Page 5
...Award for Best Biotechnology Product. Amgen was among the top 10 in both Science's 2009 Top Employers List for large companies and The Scientist's 2009 survey of the Best Places to Work in Industry. â- â- â- Amgen operates in a difï¬cult environment. Discovering and developing innovative new... -
Page 6
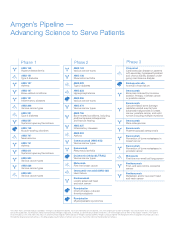
Amgen's Pipeline - Advancing Science to Serve Patients Phase 1 AMG 145 Hypercholesterolemia AMG 151 Type 2 diabetes AMG 157 Asthma AMG 167 Bone-related conditions AMG 191 Inï¬,ammatory diseases AMG 208 Various cancer types AMG 221 Type 2 diabetes AMG 557 Systemic lupus erythematosus AMG 745 Muscle-... -
Page 7
... metastatic colorectal cancer. Progressed clinical trials, with more than 35,000 patients in more than 50 countries enrolled as of year-end. Exercised option to an exclusive worldwide license (excluding Japan) to Cytokinetics' cardiac contractility program, which includes omecamtiv mecarbil, a novel... -
Page 8
...University of California, Los Angeles, in July 2009 for the third national symposium. Amgen Scholars, a $27.5-million program free education, tools, and resources available to cancer patients and their caregivers. Amgen's four nonproï¬t partners-Prevent Cancer Foundation, Cancer Support Community... -
Page 9
.... Amgen will also market denosumab for all oncology indications in Europe and other markets speciï¬ed in the agreement. GSK will register and commercialize denosumab for all indications in countries where Amgen does not currently market products, including China, Brazil, India, and South Korea... -
Page 10
... product technology rights, primarily ENBREL, related to the Immunex Corporation ("Immunex") acquisition in 2002. To exclude the net tax beneï¬ t recognized as the result of resolving certain transfer pricing issues with the Internal Revenue Service for prior periods. To exclude stock option... -
Page 11
... THE SECURITIES EXCHANGE ACT OF 1934 Commission file number 000-12477 (Exact name of registrant as specified in its charter) Delaware (State or other jurisdiction of incorporation or organization) One Amgen Center Drive, Thousand Oaks, California (Address of principal executive offices) 95-3540776... -
Page 12
......Marketed Products and Selected Product Candidates ...Marketing and Distribution ...Reimbursement ...Manufacturing, Distribution and Raw Materials ...Business Relationships ...Government Regulation ...Research and Development and Selected Product Candidates ...Human Resources ...Executive Officers... -
Page 13
..., antibodies and peptibodies) and small molecules. We maintain sales and marketing forces primarily in the United States, Europe and Canada. We market our products to healthcare providers, including physicians or their clinics, dialysis centers, hospitals and pharmacies. Most patients receiving... -
Page 14
... phase 3 head-to-head trials evaluating denosumab versus Zometa® (zoledronic acid) in the treatment of bone metastases: O in patients with advanced breast cancer, in which denosumab was superior to Zometa® in delaying the time to the first skeletal-related event ("SRE") and delaying the time to... -
Page 15
... products marketed by large pharmaceutical corporations, which may have greater clinical, research, regulatory, manufacturing, marketing, financial and human resources than we do. In addition, the introduction of new products or the development of new processes or technologies by competitors or new... -
Page 16
... plans to pursue development of biosimilars in the United States. Further, the development of new treatment options or standards of care may require less use of our products, particularly in supportive cancer care, or limit the utility and application of ongoing clinical trials for our product... -
Page 17
... to train and enroll in the ESA APPRISE (Assisting Providers and cancer Patients with Risk Information for the Safe use of ESAs) Oncology Program and to document that a discussion about the risks of ESAs took place with each patient prior to the initiation of each new course of ESA therapy. The ESA... -
Page 18
...KA"), a joint venture between Kirin Holdings Company, Limited ("Kirin") and Amgen (see "Business Relationships - Kirin Holdings Company, Limited"), to manufacture and market Aranesp® in the United States, all European countries, Canada, Australia, New Zealand, Mexico, all Central and South American... -
Page 19
... market EPOGEN® in the United States for dialysis patients. We granted Ortho Pharmaceutical Corporation, a subsidiary of J&J (which has assigned its rights under the Product License Agreement to Centocor Ortho Biotech Products), a license to commercialize recombinant human erythropoietin as a human... -
Page 20
...of cancer by managing tumor growth. We were granted an exclusive license to manufacture and market Neulasta® and NEUPOGEN® in the United States, Europe, Canada, Australia and New Zealand under a licensing agreement with KA (see "Business Relationships - Kirin Holdings Company, Limited"). We market... -
Page 21
... from companies marketing or developing treatments for neutropenia associated with chemotherapy, for bone marrow and PBPC transplant patients, severe chronic neutropenia and AML. NEUPOGEN® competes with Neulasta® in the United States and Europe. U.S. and international NEUPOGEN® sales have... -
Page 22
...molecules before they can trigger inflammation. We acquired the rights to ENBREL in July 2002 with our acquisition of Immunex Corporation ("Immunex"). We market ENBREL under a co-promotion agreement with Pfizer Inc. ("Pfizer") in the United States and Canada (see "Business Relationships - Pfizer Inc... -
Page 23
... marketed products, various companies are developing products which may compete with ENBREL in the future, including Abbott, which is developing ABT-874 in phase 3 trials for the treatment of psoriasis. Abbott has announced that they are planning to submit this indication for regulatory approval... -
Page 24
... could negatively impact product sales of Sensipar®/Mimpara®. The following table reflects companies and their currently marketed products that primarily compete with Sensipar® in the United States and Mimpara® in Europe in the nephrology segment for patients with CKD on dialysis. The table and... -
Page 25
...information, see "Research and Development and Selected Product Candidates." Based on the results of these studies, we are planning to file for regulatory approval in the United States and EU for first- and second- line treatment in patients with KRAS wild-type mCRC. Our outstanding material patents... -
Page 26
... candidates will face substantial competition from products currently marketed as well as those under development by other biotechnology and pharmaceutical companies. Denosumab Developments Denosumab is a fully human monoclonal antibody that specifically targets a ligand known as RANKL (that binds... -
Page 27
... adverse event rates that will inform the methodology of our previously submitted post-marketing surveillance program, although the letter did not require additional pre-marketing clinical trials to complete the review of the treatment indication. The FDA has also requested a new clinical program to... -
Page 28
...three studies will form the basis of the clinical evidence package for denosumab in advanced cancer, which will be submitted to regulatory authorities later in 2010. For more information, see "Research and Development and Selected Product Candidates." Patents and Competition Our outstanding material... -
Page 29
...sales. Under a co-promotion agreement, we and Pfizer market ENBREL in the United States and Canada for all approved indications. Under a co-promotion agreement with GSK, we and GSK will commercialize Amgen's Proliaâ„¢ in Europe, Australia, New Zealand and Mexico, and GSK will commercialize denosumab... -
Page 30
..., both for-profit and not-for-profit, and some companies are "self-insured" (i.e., they pay for all healthcare costs incurred by employees directly through a plan administered by a third party). Generally, employer-sponsored insurance premiums are paid primarily by employers and secondarily by... -
Page 31
...for ESRD. Under the proposed rule, the bundled payment system will include dialysis services covered under the current composite rate, as well as drugs and biologicals furnished for treatment of ESRD that are currently billed separately, including ESAs, intravenous iron and intravenous vitamin D, as... -
Page 32
... a review of all Medicare policies related to the administration of ESAs in non-renal disease applications as part of a national coverage analysis ("NCA"), which is generally CMS' first step toward developing a NCD. Subsequently, on May 14, 2007, CMS issued a proposed NCD that was open for public... -
Page 33
... upon both the clinical as well as the economic value of a product. Although the methods employed by different HTA agencies differ from country to country, the use of formal economic metrics has been increasing across Europe as well as in several emerging markets throughout the world. With increased... -
Page 34
.... These licenses generally require us to pay royalties to the licensors based on product sales. Commercial Bulk Manufacturing We operate commercial bulk manufacturing facilities in Puerto Rico and in several locations throughout the United States (see "Item 2. Properties"). Other than for ENBREL, we... -
Page 35
...-source unaffiliated third-party suppliers. Certain of these raw materials, medical devices and components are the proprietary products of these unaffiliated third-party suppliers and, in some cases, such proprietary products are specifically cited in our drug application with regulatory agencies... -
Page 36
... develops and commercializes certain of our and Kirin's product rights, which have been transferred to this joint venture. KA has given exclusive licenses to us to manufacture and market: (i) darbepoetin alfa in the United States, Europe, Canada, Australia, New Zealand, Mexico, all Central and South... -
Page 37
... States and Canada are reserved to Pfizer. Under the agreement, a management committee comprised of equal representation from Amgen and Pfizer is responsible for overseeing the marketing and sales of ENBREL, including strategic planning, the approval of an annual marketing plan, product pricing... -
Page 38
...Federal Food, Drug and Cosmetic Act ("FDCA") and the regulations promulgated thereunder, and other federal and state statutes and regulations govern, among other things, the raw materials and components used in the production of, research, development, testing, manufacture, quality control, labeling... -
Page 39
... to as PMCs or PMRs. In the United States, under the Food and Drug Administration Amendments Act of 2007 (the "FDAAA"), if the FDA becomes aware of new safety information after approval of a product, they may require us to conduct further clinical trials to assess a known or potential serious risk... -
Page 40
...manufacturing and testing of products prior to providing approval to market a product. If after receiving clearance from the FDA, we make a material change in manufacturing equipment, location or process, additional regulatory review may be required. We also must adhere to current Good Manufacturing... -
Page 41
... the Public Health Service ("PHS") drug pricing program. Under the Medicaid drug rebate program, we pay a rebate for each unit of our product reimbursed by Medicaid. The amount of the rebate for each of our products is currently set by law as a minimum 15.1% of the Average Manufacturer Price ("AMP... -
Page 42
... modality best suited to address a specific target. As such, our discovery research programs may yield targets that lead to the development of human therapeutics delivered as large molecules (such as proteins, antibodies and peptibodies) or small molecules. To execute our clinical trial programs, we... -
Page 43
... conduct clinical trials in humans before we can commercialize and sell any of our product candidates or existing products for new indications.") We have major R&D centers in several locations throughout the United States and in the United Kingdom, as well as smaller research centers in Canada and... -
Page 44
... Inflammation Hematology/Oncology General Medicine Inflammation General Medicine General Medicine Inflammation Inflammation Hematology/Oncology Hematology/Oncology Hematology/Oncology Phase 1 clinical trials investigate safety and proper dose ranges of a product candidate in a small number of human... -
Page 45
... information about selected product candidates that have advanced into human clinical trials. AMG 102 AMG 102 is a fully human monoclonal antibody that blocks the action of hepatocyte growth factor/scatter factor. It is being investigated as a cancer treatment. Phase 2 studies of single agent... -
Page 46
... review of outcomes and adverse events, including the results of TREAT (which was conducted in subjects with CKD, anemia and type 2 diabetes who were not receiving dialysis), the RED-HFâ„¢ Trial DMC recommended that the study continue as designed. Conatumumab (AMG 655) Conatumumab is a fully human... -
Page 47
... difference in the rate of ONJ between the two treatment arms. Infectious adverse events were balanced between the two treatment arms, as was OS and the time to cancer progression. In 2009, we also announced that a pivotal, phase 3, head-to-head trial evaluating denosumab administered subcutaneously... -
Page 48
... NSCLC is nearly complete. Based on current event rates, we anticipate completion of the study in 2011. In April 2009, Amgen and Millennium announced the phase 2 trial in metastatic breast cancer has been completed and the results support continued development. Nplate® (romiplostim) Nplate® is... -
Page 49
...enrollment in January 2008. Based on current event rates, we anticipate completion of the study in dialysis patients in 2011. Vectibix® (panitumumab) Vectibix® is a fully human monoclonal antibody antagonist of the EGFr pathway. It is being investigated as a cancer treatment. In September 2009, we... -
Page 50
...develop independently the same or similar information or obtain access to our information. Executive Officers of the Registrant The executive officers of the Company as of January 31, 2010 are as follows: Mr. Kevin W. Sharer, age 61, has served as a director of the Company since November 1992. Chief... -
Page 51
... as Corporate Vice President, Regulatory and Clinical Affairs and Corporate Vice President, Quality System. Mr. Robert A. Bradway, age 47, became Executive Vice President and Chief Financial Officer in April 2007. He joined the Company in 2006 as Vice President, Operations Strategy. Previously... -
Page 52
...relations, general economic conditions, geopolitical events and international operations. Further, additional risks not currently known to us or that we currently believe are immaterial also may impair our business, operations, liquidity and stock price materially and adversely. Our current products... -
Page 53
... indication of our product and we may incur increased development costs, delays in regulatory approval, associated delays in a product candidate reaching the market or the expansion of existing product labels for new indications. The occurrence of a number of high profile safety events has caused an... -
Page 54
... report to regulatory agencies side effects or other safety concerns that occur from their use of our products in clinical trials or studies or from marketed use, resulting regulatory action could adversely affect the sales of our products and our business and results of operations. If regulatory... -
Page 55
... be materially and adversely affected. Further, safety signals, trends, adverse events or results from clinical trials or studies performed by us or by others (including our licensees or independent investigators) from the marketed use of our drugs that result in revised safety-related labeling or... -
Page 56
... obtain approvals in the timeframe needed to execute our product strategies, our business and results of operations would be materially adversely affected. Additional information on our clinical trials can be found on our website at www.amgen.com. (This website address is not intended to function as... -
Page 57
... standard of medical care, limiting the utility and application of such trials. We may not obtain favorable clinical trial results and may not be able to obtain regulatory approval for new product candidates, new indications for existing products or maintenance of our current labels on this basis... -
Page 58
..., could have a material adverse impact on our business. (See "- Our current products and products in development cannot be sold if we do not gain or maintain regulatory approval.") Public and private insurers have pursued, and continue to pursue, aggressive cost containment initiatives, which... -
Page 59
... of patent litigation with generic competitors before the five year period of data exclusivity provided for under the HatchWaxman Act has expired and prior to the expiration of the patents listed for the product. Moreover, if we lose or settle current or future litigations at certain stages or... -
Page 60
... is a major risk in testing and marketing biotechnology and pharmaceutical products. We may face substantial product liability exposure in human clinical trials and for products that we sell after regulatory approval. Product liability claims, regardless of their merits, could be costly and divert... -
Page 61
...effect on our business and results of operations. Changes in credit ratings issued by nationally recognized credit rating agencies could adversely affect our cost of financing and have an adverse effect on the market price of our securities. Current economic conditions may magnify certain risks that... -
Page 62
... of our products. Certain of these raw materials, medical devices and components are the proprietary products of these unaffiliated third-party suppliers and are specifically cited in our drug application with regulatory agencies so that they must be obtained from that specific sole source and could... -
Page 63
... assist in the production of ENBREL, Sensipar®/Mimpara® and Nplate® as well as our late-stage product candidate denosumab and plan to use contract manufacturers to produce a number of our other late-stage product candidates. Our ability to adequately and timely manufacture and supply our products... -
Page 64
... of product quality testing If the efficient manufacture and supply of our products is interrupted, we may experience delayed shipments, supply constraints, stock-outs and/or recalls of our products. If we are at any time unable to provide an uninterrupted supply of our products to patients, we... -
Page 65
...new drugs currently in development, drugs currently approved for other indications that may later be approved for the same indications as those of our products and drugs approved for other indications that are used off-label. Large pharmaceutical companies and generic manufacturers of pharmaceutical... -
Page 66
... of Fresenius North America's commercial requirements for ESAs for use in managing the anemia of its hemodialysis patients in the United States and Puerto Rico, based on forecasts provided by Fresenius North America and subject to the terms and conditions of the agreement. Our marketing of ENBREL is... -
Page 67
...products. Government agencies promulgate regulations and guidelines directly applicable to us and to our products. However, professional societies, practice management groups, insurance carriers, physicians, private health/ science foundations and organizations involved in various diseases from time... -
Page 68
... applicable U.S. federal and state regulations and all potentially applicable foreign regulations and/or that we effectively manage all operational risks. The development, manufacturing, distribution, pricing, sales, marketing and reimbursement of our products, together with our general operations... -
Page 69
... Clinical: Research and/or Development Sales and Marketing denosumab Aranesp ® Location United States: Thousand Oaks, California ...Fremont, California ...San Francisco, California ...Boulder, Colorado ...Longmont, Colorado ...Washington, D.C...Louisville, Kentucky ...Cambridge, Massachusetts... -
Page 70
... all our products at our Puerto Rico manufacturing facility; if significant natural disasters or production failures occur at this facility, we may not be able to supply these products.", "- We rely on single-source third-party suppliers for certain of our raw materials, medical devices and... -
Page 71
... have been paid on the common stock to date, and we currently do not intend to pay any dividends. The following table sets forth, for the periods indicated, the range of high and low quarterly closing sales prices of the common stock as quoted on The NASDAQ Stock Market: High Year ended December 31... -
Page 72
... year. The historical stock price performance of the Company's Common Stock shown in the performance graph below is not necessarily indicative of future stock price performance. Amgen vs. Amex Biotech, Amex Pharmaceutical and S&P 500 Indices Comparison of Five Year Cumulative Total Return Value of... -
Page 73
... of factors including the stock price, blackout periods in which we are restricted from repurchasing shares, and our credit rating and may include private block purchases as well as market transactions. During the three months ended December 31, 2009, we had one outstanding stock repurchase program... -
Page 74
... to certain employees under short-term retention plans, including non-cash compensation expense associated with stock options assumed in connection with the acquisition, non-cash expense related to valuing the inventory acquired at fair value, which is in excess of our manufacturing cost, and... -
Page 75
(3) Included in Cost of sales (excludes amortization of certain acquired intangible assets) for 2007 is a charge of $30 million related to the write-off of the cost of a semi-completed manufacturing asset that will not be used due to a change in manufacturing strategy. Included in R&D expenses for ... -
Page 76
...accounted for separately. The equity components of our convertible notes, including our 2011 Convertible Notes, 2013 Convertible Notes and 2032 Modified Convertible Notes, are included in "Common stock and additional paid-in capital" in the Consolidated Balance Sheets, with a corresponding reduction... -
Page 77
... private payer healthcare programs, which are placing greater emphasis on cost containment, including requiring that the economic value of products be clearly demonstrated. Governments may regulate access to, prices or reimbursement levels of our products to control costs or to affect levels of use... -
Page 78
... cancer care, nephrology and inflammation. Our principal products include Aranesp®, EPOGEN®, Neulasta®, NEUPOGEN® and ENBREL all of which are sold in the United States. ENBREL is marketed under a co-promotion agreement with Pfizer in the United States and Canada. Our international product sales... -
Page 79
... 2008 due to improved productivity and efficiency in our capital program. We believe that existing funds, cash generated from operations and existing sources of and access to financing are adequate to satisfy our working capital, capital expenditure and debt service requirements for the foreseeable... -
Page 80
... and policies, government programs, regulatory developments or guidelines, clinical trial outcomes, clinical practice, contracting and pricing strategies, wholesaler and end-user inventory management practices, patient population growth, fluctuations in foreign currency exchange rates, new product... -
Page 81
... and private insurance plans; O O O • our ability to maintain worldwide segment share and differentiate Aranesp® from current and potential future competitive therapies or products, including J&J's Epoetin alfa product marketed in the United States and certain other locations outside of... -
Page 82
...organization regulations or guidelines relating to the use of our product; • our contracting and related pricing strategies; • severity and duration of the current global economic downturn; • development of new protocols, tests and/or treatments for cancer and/or new chemotherapy treatments or... -
Page 83
... product, regulatory or private healthcare organization medical guidelines and reimbursement practices; • our contracting and related pricing strategies; • changes in dose utilization; • development of new modalities or therapies to treat anemia associated with CRF; and • patient population... -
Page 84
... products, regulatory or private healthcare organization medical guidelines and reimbursement practices; • severity and duration of the current global economic downturn; • our contracting and related pricing strategies; • expansion into new international territories; and • patient population... -
Page 85
... governmental or private organization regulations or guidelines relating to the use of our product; • cost containment pressures from governments and private insurers on healthcare providers; • our contracting and related pricing strategies; and • patient population growth. See "Item 1A. Risk... -
Page 86
.... Research and development R&D costs are expensed as incurred and primarily include salaries, benefits and other staff-related costs; facilities and overhead costs; clinical trial and related clinical manufacturing costs; contract services and other outside costs; information systems' costs and... -
Page 87
... of $32 million, expense recoveries associated with our GSK collaboration agreement for Proliaâ„¢ in PMO in Europe, Australia, New Zealand and Mexico of $29 million, lower staff-related costs of $28 million, lower global enterprise resource planning ("ERP") system related expenses of $28 million and... -
Page 88
...the impairment of a non-ENBREL related intangible asset previously acquired in the Immunex acquisition. Write-off of acquired in-process research and development In accordance with the accounting standards for business combinations, prior to January 1, 2009, the fair value of acquired IPR&D projects... -
Page 89
... R&D expenses. Panitumumab was Abgenix's fully human monoclonal antibody which, at acquisition, was in phase 2/3 clinical trials for the treatment of certain types of cancer. The incremental R&D expenses assumed to be incurred to obtain necessary regulatory approvals for the various indications of... -
Page 90
... consolidated results of operations, financial position or cash flows. In October 2009, the FASB issued a new accounting standard which amends guidance on accounting for revenue arrangements involving the delivery of more than one element of goods and/or services. This standard addresses the unit of... -
Page 91
... through a variety of sources, including: cash provided by operating activities, sale of marketable securities, borrowings through commercial paper and/or our syndicated credit facility and access to other debt markets and equity markets. (See "Item 1A. Risk Factors - Current economic conditions may... -
Page 92
...the occurrence of a change in control triggering event, as defined, we may be required to purchase for cash all or a portion of the 2019 Notes and the 2039 Notes at a price equal to 101% of the principal amount of the notes plus accrued interest. Debt issuance costs totaled approximately $13 million... -
Page 93
... facility which matures in November 2012 and is available for general corporate purposes or as a liquidity backstop to our commercial paper program. Annual commitment fees for this facility are 0.045% based on our current credit rating. As of December 31, 2009, no amounts were outstanding under... -
Page 94
... capacity expansions in Puerto Rico, Fremont and other site developments and investment in our global ERP system and other information systems' projects. Capital expenditures in 2007 were primarily associated with manufacturing capacity and site expansions in Puerto Rico and other locations and... -
Page 95
... of employee stock options will vary from period to period based upon, among other factors, fluctuations in the market value of our stock relative to the exercise price of such options. Off-Balance Sheet Arrangements We do not have any off-balance sheet arrangements that are material or reasonably... -
Page 96
... (including those related to clinical trials) for new and existing products; (iii) capital expenditures; (iv) open purchase orders for the acquisition of goods and services in the ordinary course of business and (v) our agreement with International Business Machines Corporation ("IBM"), which... -
Page 97
... to be claimed on the related sales. These estimates take into consideration current contractual and statutory requirements, specific known market events and trends, internal and external historical data and forecasted customer buying patterns. Sales deductions are substantially product-specific and... -
Page 98
... risks associated with the manufacturing of the product candidate as well as considering the remaining shelf life of the inventory in relation to the expected launch date. Upon capitalization, we continue to monitor any changes in these factors. In the event of any significant negative developments... -
Page 99
... RISK We are a global biotechnology company with operations in various countries. We are exposed to market risks that may result from changes in interest rates, foreign currency exchange rates and prices of equity instruments as well as changes in the general economic conditions in the countries... -
Page 100
... of operations are affected by fluctuations in the value of the U.S. dollar as compared to foreign currencies, predominately the Euro, as a result of the sale of our products in foreign markets. Increases and decreases in our international product sales from movements in foreign exchange rates are... -
Page 101
... in Amgen's Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms, and that such information is accumulated and communicated to Amgen's management, including its Chief Executive Officer and Chief Financial Officer, as... -
Page 102
... 2009, based on those criteria. The effectiveness of the Company's internal control over financial reporting has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report appearing below, which expresses an unqualified opinion on the effectiveness... -
Page 103
...opinion, Amgen Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2009, based on the COSO criteria. We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Consolidated... -
Page 104
... a code of ethics applicable to our principal executive officer, principal financial officer, principal accounting officer or controller, and other persons performing similar functions. To view this code of ethics free of charge, please visit our website at www.amgen.com (This website address is not... -
Page 105
... OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS Securities Authorized for Issuance Under Existing Equity Compensation Plans The following table sets forth certain information as of December 31, 2009 concerning our common stock that may be issued under any form of award granted under all... -
Page 106
... December 31, 2009. Such purchases reflect 95% of the closing price of the Common Stock on the applicable Purchase Date. These plans have terminated as to future grants. These Plans were originally assumed pursuant to the terms of the merger agreement between Amgen and Immunex which was approved by... -
Page 107
... and management is incorporated by reference from the sections entitled "SECURITY OWNERSHIP OF DIRECTORS AND EXECUTIVE OFFICERS and SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS" in our Proxy Statement. Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE Information... -
Page 108
...Annual Report: II. Valuation Accounts ... Page number F-58 All other schedules are omitted because they are not applicable, not required or because the required information... of Designations of the Series A Junior Participating Preferred Stock (As Effective December 10, 2008). (Filed as an... -
Page 109
... which are dated December 15, 2008, replaces the current trustee under the agreements listed as Exhibits 4.9 and 4.16, respectively, with Bank of New York Mellon. Amgen Inc. hereby agrees to furnish copies of these agreements to the Securities and Exchange Commission upon request. First Supplemental... -
Page 110
... Rights Agreement, dated as of May 30, 2007, among Amgen Inc. and Morgan Stanley & Co. Incorporated, Merrill Lynch, Pierce, Fenner & Smith Incorporated, Barclays Capital Inc., Credit Suisse Securities (USA) LLC, Goldman, Sachs & Co., Citigroup Global Markets Inc., J.P. Morgan Securities Inc... -
Page 111
...2007 and incorporated herein by reference.) Product License Agreement, dated September 30, 1985, and Technology License Agreement, dated September 30, 1985, between Kirin-Amgen, Inc. and Ortho Pharmaceutical Corporation. (Filed as an exhibit to Form 10-Q for the quarter ended June 30, 2000 on August... -
Page 112
...22, 2002 and incorporated herein by reference.) Amended and Restated Promotion Agreement, dated as of December 16, 2001, by and among Immunex Corporation, American Home Products Corporation and Amgen Inc. (with certain confidential information deleted therefrom). (Filed as an exhibit to Amendment No... -
Page 113
.... and Merrill Lynch International. (Filed as an exhibit to Form 10-Q for the quarter ended June 30, 2007 on August 9, 2007 and incorporated herein by reference.) Collaboration Agreement, dated July 11, 2007, between Amgen Inc. and Daiichi Sankyo Company (with certain confidential information deleted... -
Page 114
... 11, 2009, to Master Services Agreement, dated October 22, 2009, between Amgen Inc. and International Business Machines Corporation (with certain confidential information deleted therefrom). Integrated Facilities Management Services Agreement, dated February 4, 2009 between Amgen Inc. and Jones Lang... -
Page 115
... the Securities Exchange Act of 1934, the registrant has duly caused this Annual Report to be signed on its behalf by the undersigned, thereunto duly authorized. AMGEN INC. (Registrant) Date: 03/01/2010 By: /S/ ROBERT A. BRADWAY Robert A. Bradway Executive Vice President and Chief Financial Officer... -
Page 116
.../S/ KEVIN W. SHARER Kevin W. Sharer Chairman of the Board, Chief Executive Officer and President, and Director (Principal Executive Officer) Executive Vice President and Chief Financial Officer (Principal Financial Officer) Vice President Finance and Chief Accounting Officer (Principal Accounting... -
Page 117
Signature Title Date /S/ GILBERT S. OMENN Gilbert S. Omenn Director 03/01/2010 /S/ JUDITH C. PELHAM Judith C. Pelham Director 03/01/2010 /S/ J. PAUL REASON J. Paul Reason Director 03/01/2010 /S/ LEONARD D. SCHAEFFER Leonard D. Schaeffer Director 02/17/2010 105 -
Page 118
... and Restated Employee Stock Purchase Plan; • Registration Statements (Form S-8 No. 33-39104, as amended by Form S-8 No. 333-144581) pertaining to the Amended and Restated Amgen Retirement and Savings Plan (formerly known as the Amgen Retirement and Savings Plan); • Registration Statements... -
Page 119
... herein by reference and our report included in the preceding paragraph with respect to the financial statement schedule of Amgen Inc. included in this Annual Report (Form 10-K) of Amgen Inc. for the year ended December 31, 2009. /s/ Ernst & Young LLP Los Angeles, California March 1, 2010 107 -
Page 120
[THIS PAGE INTENTIONALLY LEFT BLANK] -
Page 121
... as a whole, presents fairly in all material respects the information set forth therein. We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Amgen Inc.'s internal control over financial reporting as of December 31, 2009, based on... -
Page 122
...: Product sales ...Other revenues ...Total revenues ...Operating expenses: Cost of sales (excludes amortization of certain acquired intangible assets presented below) ...Research and development ...Selling, general and administrative ...Amortization of certain acquired intangible assets ...Write-off... -
Page 123
... ...Marketable securities ...Trade receivables, net ...Inventories ...Other current assets ...Total current assets ...Property, plant and equipment, net ...Intangible assets, net ...Goodwill ...Other assets ...Total assets ...LIABILITIES AND STOCKHOLDERS' EQUITY Current liabilities: Accounts payable... -
Page 124
...stock in connection with the Company's equity award programs ...Stock-based compensation ...Tax impact related to employee stock options ...Repurchases of common stock... of required retrospective adoption of a new accounting standard effective January 1, 2009, applicable to our convertible debt. F-4 -
Page 125
...) 688 Deferred revenue ...33 327 52 Net cash provided by operating activities ...Cash flows from investing activities: Purchases of property, plant and equipment ...Cash paid for acquisitions, net of cash acquired ...Purchases of marketable securities ...Proceeds from sales of marketable securities... -
Page 126
... 31, 2009 1. Summary of significant accounting policies Business Amgen Inc. (including its subsidiaries, referred to as "Amgen," "the Company," "we," "our" and "us") is a global biotechnology medicines company that discovers, develops, manufactures and markets medicines for grievous illnesses. We... -
Page 127
.... Research and development costs R&D costs are expensed as incurred and primarily include salaries, benefits and other staff-related costs; facilities and overhead costs; clinical trial and related clinical manufacturing costs; contract services and other outside costs; information systems' costs... -
Page 128
... rather than completed, all capitalized amounts are written-off immediately. Share based payments We have employee compensation plans under which various types of stock-based instruments are granted. All share-based payments to employees, including grants of employee stock options, are recognized in... -
Page 129
... standard did not have a material impact on our consolidated results of operations, financial position or cash flows. Effective April 1, 2009, we adopted a new accounting standard that provides additional guidance in estimating fair value when the market volume and level of activity for an asset... -
Page 130
... Inventories are stated at the lower of cost or market. Cost, which includes amounts related to materials, labor and overhead, is determined in a manner which approximates the first-in, first-out ("FIFO") method. The Company capitalizes inventories produced in preparation for product launches when... -
Page 131
... consolidated results of operations, financial position or cash flows. In October 2009, the FASB issued a new accounting standard which amends guidance on accounting for revenue arrangements involving the delivery of more than one element of goods and/or services. This standard addresses the unit of... -
Page 132
AMGEN INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued) The following tables illustrate the impact of adopting this accounting standard on our Consolidated Statements of Income (in millions, except per share information... originally accounting reported standard "Revised" Operating income ... -
Page 133
... comprised of marketing rights to marketed products, based on their estimated fair values at the acquisition date and the excess of the purchase price over the fair values of net assets acquired of approximately $105 million was assigned to goodwill. There was no material gain or loss related to the... -
Page 134
... specialized in the development of drugs for the treatment of diabetes and inflammatory diseases. Pursuant to the merger agreement, we paid cash of approximately $300 million to acquire all of the outstanding shares of Alantos. The purchase price paid, including transaction costs, was allocated to... -
Page 135
... of grant. Eligible employees generally receive a grant of stock options and/or restricted stock units annually with the number of shares and type of instrument generally determined by the employee's salary grade and performance level. In addition, certain management and professional level employees... -
Page 136
... options. The risk-free interest rates for periods within the expected life of the option are based on the U.S. Treasury yield curve in effect during the period the options were granted. Stock option information with respect to our stock-based compensation plans during the three years ended December... -
Page 137
... compensation cost related to nonvested awards of both stock options and restricted stock units, which is expected to be recognized over a weighted-average period of 1.7 years. Performance award program Certain management-level employees also receive annual grants of performance units, which give... -
Page 138
... period and compound annual growth rate goals for total stockholder return based on the provisions of the award. As of December 31, 2009, there was approximately $38 million of total estimated unrecognized compensation cost related to the 2009 and 2008 performance unit grants that is expected... -
Page 139
... December 31, 2009 2008 Deferred tax assets: Intercompany inventory related items ...Expense accruals ...Acquired net operating loss and credit carryforwards ...Expenses capitalized for tax ...Stock-based compensation ...Deferred revenue ...Other ...Total deferred tax assets ...Valuation allowance... -
Page 140
... the IRS for certain matters, primarily related to transfer pricing tax positions, for the years ended December 31, 2005 and 2006 and have remeasured our UTBs accordingly. Also during 2009, we settled the examination of our California state income tax returns for certain matters for the years ended... -
Page 141
...respectively. These earnings include income from manufacturing operations in Puerto Rico under tax incentive grants that expire in 2020. One or more of our legal entities file income tax returns in the U.S. federal jurisdiction, various U.S. state jurisdictions and certain foreign jurisdictions. Our... -
Page 142
... States and Canada are reserved to Pfizer. Under the agreement, a management committee comprised of equal representation from Amgen and Pfizer is responsible for overseeing the marketing and sales of ENBREL, including strategic planning, the approval of an annual marketing plan and product pricing... -
Page 143
... costs of these products in Japan and we will receive royalties on future sales of these products in Japan. Amgen has the right to participate in the promotion of the products in Japan. In February 2008, we also entered into a collaboration agreement with Takeda for the worldwide development... -
Page 144
... Array in December 2009, which granted us exclusive worldwide rights to Array's small-molecule glucokinase activator program, including ARRY-403, currently being tested in a phase 1 clinical trial in patients with Type 2 diabetes. In connection with entering the agreement, we paid Array $60 million... -
Page 145
...restructure our worldwide operations in order to improve our cost structure. This restructuring plan was primarily the result of regulatory and reimbursement developments that began in 2007 involving erythropoiesis-stimulating agent ("ESA") products, including our marketed ESA products Aranesp® and... -
Page 146
... (Continued) saving initiatives, $19 million of other charges and $10 million loss on the disposal of certain less significant marketed products, offset by $115 million of cost recoveries from Pfizer. The following tables summarize the charges (credits) related to the above-noted actions by type of... -
Page 147
... manufacturing operation. Other restructuring charges incurred in 2009 primarily relate to integration costs associated with our cost savings initiatives and loss accruals for certain leases that will not be used in our business. Integration costs totaled $32 million and are included in "Research... -
Page 148
... Amortized cost Gross unrealized gains Gross unrealized losses Estimated fair value Type of security: U.S. Treasury securities ...Obligations of U.S. government agencies and FDIC guaranteed bank debt ...Corporate debt securities ...Mortgage and asset backed securities ...Money market mutual funds... -
Page 149
...money market instruments issued by institutions primarily with investment grade credit ratings and places restrictions on maturities and concentration by type and issuer. We review our available-for-sale securities for other-than-temporary declines in fair value below their cost basis on a quarterly... -
Page 150
AMGEN INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued) During 2008, we wrote-off $84 million of inventory resulting from a strategic decision to change manufacturing processes. This charge is included in "Cost of sales (excludes amortization of certain acquired intangible assets)" in our ... -
Page 151
... 31, 2009 2008 Sales deductions ...Employee compensation and benefits ...Clinical development costs ...Sales returns reserve ...Other ... $ 970 751 361 211 1,006 $3,299 $ 876 799 429 233 1,045 $3,382 16. Financing arrangements The following table reflects the carrying value of our long-term... -
Page 152
... rates will be adjusted if we make specified types of distributions or enter into certain other transactions with respect to our common stock. The 2011 Convertible Notes and 2013 Convertible Notes may only be converted: (i) during any calendar quarter if the closing price of our common stock... -
Page 153
... the discount resulting from the adoption of the new accounting standard. The remaining balance of the interest expense relates to the contractual coupon rates. For the years ended December 31, 2009, 2008 and 2007, total interest expense for the 2013 Convertible Notes was $127 million, $120 million... -
Page 154
...the occurrence of a change in control triggering event, as defined, we may be required to purchase for cash all or a portion of the 2019 Notes and the 2039 Notes at a price equal to 101% of the principal amount of the notes plus accrued interest. Debt issuance costs totaled approximately $13 million... -
Page 155
... facility which matures in November 2012 and is available for general corporate purposes or as a liquidity backstop to our commercial paper program. Annual commitment fees for this facility are 0.045% based on our current credit rating. As of December 31, 2009, no amounts were outstanding under... -
Page 156
... resulting from the adoption of the new accounting standard that changed the method of accounting for our convertible debt. (See above and Note 2, "Change in method of accounting for convertible debt instruments.") 17. Stockholders' equity Stock repurchase program A summary of the activity under our... -
Page 157
... repurchased will vary based on a variety of factors, including the stock price, blackout periods in which we are restricted from repurchasing shares, and our credit rating and may include private block purchases as well as market transactions. Accumulated other comprehensive income The components... -
Page 158
... that reflect the Company's assumptions about the inputs that market participants would use in pricing the asset or liability and are developed based on the best information available in the circumstances. The fair value hierarchy is broken down into three levels based on the source of inputs as... -
Page 159
...prices in Significant active markets for Significant other unobservable identical assets observable inputs inputs (Level 1) (Level 2) (Level 3) Total Assets: Available-for-sale securities: U.S. Treasury securities ...Obligations of U.S. government agencies and FDIC guaranteed bank debt ...Corporate... -
Page 160
... 1,017 994 948 536 567 58 111 $9,751 The Company is exposed to certain risks related to its business operations. The primary risks that we manage by using derivative instruments are foreign exchange rate risk and interest rate risk. We use financial instruments, including foreign currency forward... -
Page 161
...Euros. Increases or decreases in the cash flows associated with our international product sales due to movements in foreign currency exchange rates are partially offset by the corresponding increases and decreases in our international operating expenses resulting from these foreign currency exchange... -
Page 162
... a desired mix of fixed and floating interest rate debt, we have entered into interest rate swap agreements, which qualify and have been designated as fair value hedges. The terms of these interest rate swap agreements correspond to the related hedged debt instruments and effectively convert a fixed... -
Page 163
... that the importation, use, sale or offer to sell pegylated erythropoietin (alternatively referred to as peg-EPO or MIRCERA®) infringes Amgen's EPO patents. Amgen alleged infringement of six of its U.S. Patents that claim erythropoietin products, pharmaceutical compositions and processes for making... -
Page 164
.... Human Genome Sciences Litigations On November 30, 2007, Human Genome Sciences ("HGS") filed an action under 35 U.S.C. §146 against Amgen in the Delaware District Court to review a Decision on Motions entered on July 26, 2007 and the Final Judgment entered November 20, 2007 by the Board of Patent... -
Page 165
... with methotrexate. Average Wholesale Price ("AWP") Litigation Amgen and Immunex are named as defendants, either separately or together, in numerous civil actions broadly alleging that they, together with many other pharmaceutical manufacturers, reported prices for certain products in a manner that... -
Page 166
... relating to motions to dismiss the complaints, discovery, class certification, summary judgment and other pre-trial issues. For the private class action cases, the Massachusetts District Court has divided the defendant companies into a Track I group and a Track II group. Both Amgen and Immunex... -
Page 167
... was filed against Amgen and Immunex on March 8, 2005, in the Supreme Court of New York, Erie County. The complaint alleges that all defendants participated in a scheme to market the spread between the true wholesale price (i.e., selling price) and the false and inflated AWP reported, in order to... -
Page 168
... product pricing information reported to the state by falsely inflating those prices. A hearing on defendants' motion to dismiss occurred on March 5, 2009, following which the court denied the motion. Federal Securities Litigation - In re Amgen Inc. Securities Litigation The six federal class action... -
Page 169
... California Corporations Code. Plaintiffs allege that the State Defendants failed to disclose and/or misrepresented results of Aranesp® clinical studies, marketed both Aranesp® and EPOGEN® for off-label uses and that these actions or inactions caused shareholders to suffer damages. The complaints... -
Page 170
... Board members breached their fiduciary duties by failing to inform current and former employees who participated in the Amgen Retirement and Savings Manufacturing Plan and the Amgen Savings Plan of the alleged off-label promotion of both Aranesp® and EPOGEN® while a number of studies allegedly... -
Page 171
... that Amgen promoted EPOGEN® and Aranesp® for: treating cancer patients who are not on chemotherapy; treating quality of life symptoms associated with anemia, such as fatigue; and reaching hemoglobin targets above the FDA-approved level. Each plaintiff asserts claims under California's consumer... -
Page 172
... public on or about May 7, 2009. The filing states that the relator in the Massachusetts Qui Tam Action is a former Amgen employee. Further, the filing represents that, in addition to the Massachusetts Qui Tam Action, there are currently nine other actions under the False Claim Act ("Qui Tam Actions... -
Page 173
... from the Attorney General of the State of New York seeking documents related to Amgen's promotional activities, sales and marketing activities, medical education, clinical studies, pricing and contracting, license and distribution agreements and corporate communications. Amgen continues to fully... -
Page 174
AMGEN INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued) Commitments We lease certain administrative, R&D, sales and marketing and manufacturing facilities and equipment under non-cancelable operating...2013 ...2014 ...Thereafter ...Total ...Less income from subleases ...Net minimum operating ... -
Page 175
...) 21. Segment information We operate in one business segment - human therapeutics. Therefore, results of our operations are reported on a consolidated basis for purposes of segment reporting, consistent with internal management reporting. Enterprise-wide disclosures about product sales, revenues and... -
Page 176
...: United States ...Puerto Rico ...International countries ...Total long-lived assets ...Major customers $ 3,525 1,920 293 $ 5,738 $ 3,836 1,740 303 $ 5,879 In the United States, we sell primarily to wholesale distributors of pharmaceutical products. We utilize these wholesale distributors as... -
Page 177
... in our business, staff separation costs and certain cost saving initiatives associated with our restructuring plan. We recorded a charge of $84 million ($64 million, net of tax) related to the write-off of inventory resulting from a strategic decision to change manufacturing processes. We recorded... -
Page 178
SCHEDULE II AMGEN INC. VALUATION ACCOUNTS Years ended December 31, 2009, 2008 and 2007 (In millions) Balance at beginning of period Additions charged to costs and expenses Other additions Balance at end of period Allowance for doubtful accounts Deductions Year ended December 31, 2009 ...Year ... -
Page 179
... Vice President, Global Commercial Operations Roger M. Perlmutter Executive Vice President, Research and Development Anna S. Richo Senior Vice President and Chief Compliance Ofï¬cer David J. Scott Senior Vice President, General Counsel and Secretary Kevin W. Sharer Chairman of the Board, CEO and... -
Page 180
... Inc. One Amgen Center Drive Thousand Oaks, CA 91320-1799 www.amgen.com About Amgen Amgen discovers, develops, manufactures, and delivers innovative human therapeutics. A biotechnology pioneer since 1980, Amgen was one of the first companies to realize the new science's promise by bringing safe...