Amgen 2006 Annual Report Download - page 7
Download and view the complete annual report
Please find page 7 of the 2006 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
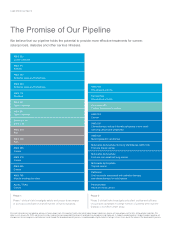
Phase 3
Phase 3 clinical trials investigate the safety and efficacy of a
product candidate in a large number of patients who have the
disease or condition under study.
Approved
Approved therapies are available for prescribed uses to patients in
countries that have granted regulatory clearance. Amgen continues to
develop many of its approved therapies for potential new indications.
This table and the Annual Report in which it appears contain forward looking statements that involve significant risks and uncertainties, including those discussed here and others that can be found in
Amgen’s most recent Form 10-K and in Amgen’s periodic reports on Form 10-Q and Form 8-K, and actual results may vary materially. Amgen is providing this information as of January 25, 2007, and
does not undertake any obligation to update any forward looking statements contained in this table or this Annual Report as a result of new information, future events or otherwise.
Denosumab
Postmenopausal osteoporosis
Cinacalcet HCI
Cardiovascular disease in patients with secondary
hyperparathyroidism and chronic kidney disease
undergoing maintenance dialysis
Darbepoetin alfa
Anemia in heart failure
Darbepoetin alfa
Cardiovascular disease in patients with
chronic kidney disease and type 2 diabetes
AMG 531
Immune thrombocytopenic purpura
(an autoimmune bleeding disorder)
Denosumab
Bone loss induced by hormone ablation therapy
for breast cancer or prostate cancer
Denosumab
Prevention of bone metastases
Denosumab
Prevention of cancer-related bone damage
Panitumumab
First- and second-line colorectal cancer
Enbrel® (etanercept)
Ankylosing spondylitis (arthritis of the spine)
ENBREL
Chronic moderate-to-severe plaque psoriasis
ENBREL
Moderate-to-severe juvenile rheumatoid arthritis
ENBREL
Moderate-to-severe rheumatoid arthritis
ENBREL
Psoriatic arthritis
Kineret® (anakinra)
Moderate-to-severe rheumatoid arthritis
Sensipar® (cinacalcet HCI)
Hypercalcemia of parathyroid carcinoma
Sensipar®
Secondary hyperparathyroidism in end-stage renal disease
Aranesp® (darbepoetin alfa)
Anemia of chronic renal disease
EPOGEN® (Epoetin alfa)
Anemia of end-stage renal disease
Aranesp®
Chemotherapy-induced anemia
Kepivance® (palifermin)
Severe oral mucositis in patients with hematologic cancers
undergoing bone marrow transplant
Neulasta® (pegfilgrastim)
Chemotherapy-induced neutropenia
NEUPOGEN® (Filgrastim)
Neutropenia (multiple indications)
Vectibix™ (panitumumab)
Metastatic colorectal cancer with disease progression
on or following standard chemotherapy
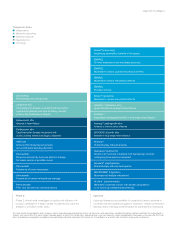
Therapeutic Areas
Inflammation
Metabolic disorders
General medicine
Neuroscience
Oncology
Amgen 2006 Annual Report 5