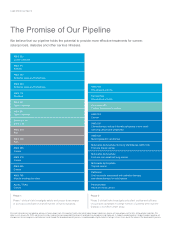
Amgen 2006 Annual Report Download - page 5
Download and view the complete annual report
Please find page 5 of the 2006 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Amgen 2006 Annual Report 3
Strategic acquisitions and partnerships also have helped us
grow. In 2006 we completed our acquisition of Abgenix, bringing
us full ownership of Vectibix™
. We acquired Avidia, obtaining a
completely novel protein platform that we’re excited about. At the
end of the year, Amgen entered into a strategic partnership with
Cytokinetics for a promising cardiovascular program.
We are carrying out the largest-scale manufacturing expansion
in the biotechnology industry. Our Puerto Rico facility recently
added two new manufacturing plants, and further expansion
is underway. In County Cork, Ireland, a planned major new
manufacturing site will help us meet demand for our medicines
in Europe and elsewhere. These investments are testaments to
our faith in our pipeline and our commitment to deliver vital
medicines to every patient, every time.
New competitive challenges
While we are proud of all that we achieved in the past year, we
are focused on new competitive challenges in 2007. Roche has
announced plans to launch a peg-EPO product in the United
States. We have compelling proof that peg-EPO violates Amgen’s
patents, and we look forward to presenting our case in court.
Our company has an enviable track record of upholding our
intellectual property rights and a robust patent estate that we
intend to vigorously defend. However, some analysts expect an
overhang effect on our stock price until this case is resolved.
In addition, we are preparing to face competition from bio-
similars in Europe for the first time. Biosimilars, or follow-on
biologics as they are called in the U.S., are not in any way
comparable to generic pharmaceutical products. Protein-based
medicines cannot be copied in the way that small molecules can
be. Their production is complex, and their safety must be ensured
through rigorous processes and tests. Amgen welcomes the
availability of additional treatment options for patients. The new
biosimilars guidelines in Europe emphasize patient safety and
sound science, and we are ready to compete on those grounds.
Doing the right thing for patients
Over the past year we’ve seen enormous progress in our pipeline
but also some setbacks in a few clinical trials. That is the nature
of science when you attempt to address serious unmet medical
needs. We tackle difficult medical and scientific challenges
because we are committed to making a dramatic difference for
patients. For the same reason, Amgen promptly discloses our
clinical results, whether good or bad. We believe transparency
is in the best interest of patients and the best way to advance
science. We chart the course, because we are confident that
our incredibly rich and strong pipeline will deliver new medicines
to restore hope for patients facing grievous illness.
Recently released trial data has raised some complex questions
about safe hemoglobin levels for patients receiving erythropoietic
products, including EPOGEN® and Aranesp®
. We are working with
medical experts and regulatory authorities to help ensure that
treatment decisions are made based on sound science. Clinical
data collected over many years and real-world experience show
that EPOGEN® and Aranesp®
, when used by doctors according
to their approved labeling, are safe and improve patients’ lives.
Further analysis and additional information should give a more
comprehensive sense of the best ways to use these medicines
to maximize safety and therapeutic benefit in different patient
populations. Amgen will continue to work with caregivers and
policy makers to share data and knowledge, to work toward the
best possible standards of care.
Promise for the future
Our challenges are substantial, but our opportunities are far
greater. Amgen has many exciting programs in oncology;
Vectibix™ is our first cancer therapeutic, and we expect many
more will follow. Our scientists are pursuing a number of
promising programs in new disease areas, from osteoporosis
to diabetes to Alzheimer’s disease. If and when these programs
lead to new therapeutic candidates, we have the proven
expertise to develop, manufacture and deliver them to patients.
Our capabilities in biotechnology are unmatched.
We have many other assets to draw upon. Amgen has strong
relationships with physicians, patient groups, scientific leaders, and
key decision makers across the health care field. We also have
a unique strength in our values-based culture. Amgen people
point to the company’s values as the reason they joined us and
the reason they stay. In our research and clinical trial enrollment
and execution, we hold ourselves and our partners to the highest
standards of ethical behavior. We follow responsible sales and
marketing practices and treat our customers with courtesy and
respect. Our leaders are thorough, transparent and principled in
governance, financial accounting and communications.
Most importantly, Amgen has a great mission—to serve
patients—and more than 20,000 great people to follow through
on that mission. I’m proud of our financial record: Over the past
five years, few if any independent public companies have
matched Amgen’s performance in compound revenue or earnings
growth. But I’m even prouder of what we have done for patients.
In the past five years, we’ve brought out seven new medicines,
all of them addressing serious unmet medical needs. I believe
that over the next five to ten years, we are going to do even
greater things to help millions of people fight grievous illness
and live more fulfilling lives.
KEVIN W. SHARER
Chairman and Chief Executive Officer
February 12, 2007