Johnson and Johnson 2013 Annual Report Download - page 7
Download and view the complete annual report
Please find page 7 of the 2013 Johnson and Johnson annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
2013 Business Highlights
Johnson & Johnson delivered strong results in 2013 led by the outstanding performance in our Pharmaceutical
business, the re-launch and strength of key brands in our U.S. over-the-counter (OTC) and other Consumer
businesses and continued progress in integrating Synthes, Inc. into our Medical Devices and Diagnostics (MD&D)
segment. Results also included advances in our longer-term growth drivers including bringing innovative
solutions to the global health care market, executing with excellence, and leading with purpose to advance health
and well-being for patients and consumers around the world.
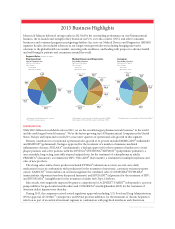
Pharmaceutical
Sales by Therapeutic Area
2013 Sales: $28.1 billion
Sales Change:
Total: 10.9%
Operational*: 12.0%
Segment Sales (in billions of dollars)
* Operational excludes the impact of currency.
** Rounded for visual accuracy.
INFECTIOUS DISEASES
$3.5**
11.1%
ONCOLOGY
$3.8
43.5%
OTHER
$4.9
0.2%
NEUROSCIENCE
$6.7
(0.8%)
IMMUNOLOGY
$9.2
16.7%
Medical Devices and Diagnostics
Sales by Major Franchise
2013 Sales: $28.5 billion
Sales Change:
(1)
Total: 3.9%
Operational*: 6.1%
ORTHOPAEDICS
(1)
$9.5
21.9%
SPECIALTY SURGERY
$2.6
2.6%
SURGICAL CARE
$6.3
(3.3%)
VISION CARE
$2.9
(2.0%)
DIABETES CARE
$2.3
(11.7%)
CARDIOVASCULAR
CARE
$2.1
4.6%
DIAGNOSTICS
$1.9
(8.9%)
INFECTION
PREVENTION/OTHER
$0.9
(4. 2% )
(1) Excluding the net impact of the Synthes acquisition, MD&D
total change = (2.1%) and Orthopaedics total change = 0.7%
(2) Nutritionals is now included in “Wound Care/Other.”
Consumer
Sales by Major Franchise
2013 Sales: $14.7 billion
Sales Change:
Total: 1.7%
Operational*: 2.8%
OTC
(2)
$4.0
7.0 %
SKIN CARE
$3.7
2.4%
WOMEN’S HEALTH
$1.6
(3.5%)
WOUND CARE/
OTHER
(2)
$1.5
(5.1%)
BABY CARE
$2.3
1.8%
ORAL CARE
$1.6
(0.1%)
PHARMACEUTICAL
With $28.1 billion in worldwide sales in 2013, we are the seventh-largest pharmaceuticals business* in the world
and the sixth-largest biotech business*. We’re the fastest-growing top 10 Pharmaceutical Company in the United
States, Europe and Japan and recorded 15 consecutive quarters of operational sales growth in this segment.
Primary contributors to exceptional operational sales growth of 12 percent included REMICADE®(infliximab)
and SIMPONI®(golimumab), biologics approved for the treatment of a number of immune-mediated
inflammatory diseases; STELARA®(ustekinumab), a biologic approved for the treatment of moderate to severe
plaque psoriasis and active psoriatic arthritis; INVEGA®SUSTENNA®/XEPLION®(paliperidone palmitate), a
once-monthly, long-acting, injectable atypical antipsychotic for the treatment of schizophrenia in adults;
PREZISTA®(darunavir), a treatment for HIV; VELCADE®(bortezomib), a treatment for multiple myeloma; and
sales of new products.
The strong sales results of new products included ZYTIGA®(abiraterone acetate), an oral, once-daily
medication for use in combination with prednisone for the treatment of metastatic, castration-resistant prostate
cancer; XARELTO®(rivaroxaban), an oral anticoagulant; the combined sales of COMPLERA®/EVIPLERA®
(emtricitabine /rilpivirine/tenofovir disoproxil fumarate) and EDURANT®(rilpivirine) for the treatment of HIV;
and INVOKANA®(canagliflozin) for the treatment of adults with Type 2 diabetes.
Sales results were negatively impacted by generic competition for ACIPHEX®/ PARIET®(rabeprazole), a proton
pump inhibitor for gastrointestinal disorders and CONCERTA®(methylphenidate HCI) for the treatment of
attention deficit hyperactivity disorder.
During 2013, the company received several regulatory approvals including: U.S. Food and Drug Administration
(FDA) approval of OLYSIO™(simeprevir), an NS3/4A protease inhibitor, for the treatment of chronic hepatitis C
infection as part of an antiviral treatment regimen in combination with pegylated interferon and ribavirin in
2013 BUSINESS HIGHLIGHTS