Eli Lilly 2013 Annual Report Download - page 6
Download and view the complete annual report
Please find page 6 of the 2013 Eli Lilly annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
4
medicines. We believe no other company will be better
positioned to meet the needs of people with diabetes across
the treatment spectrum. (See page 8.)
In January 2011, Lilly entered into a global alliance
with Boehringer Ingelheim to jointly develop and com-
mercialize two new oral diabetes therapies: Trajenta®, the
oral DPP-4 inhibitor we launched in 2011—now approved
in more than 60 countries—and empagliozin, an SGLT-2
inhibitor. e Lilly-BI partnership also includes Lilly’s new
insulin glargine product.
In addition to the products in the
alliance, Lilly is advancing our basal
insulin peglispro and our GLP-1 receptor
agonist, dulaglutide—further expanding
the potential reach of our portfolio.
Even as we continue to deliver solid
performance with our marketed products,
we can take full advantage of our existing
commercial footprint as we launch as
many as four new diabetes medicines in
the next two to three years.
Empagliozin was submitted for
regulatory approval in the U.S., Europe,
and Japan in 2013. We’re encouraged by the
data from three Phase III studies which all
met their primary objectives. We observed
statistically signicant reductions in HbA1c,
a measure of average blood glucose, and
we also saw decreases in body weight and
reductions in systolic blood pressure.
Dulaglutide was submitted in late 2013 in the U.S. and
Europe. We’ve completed six Phase III trials; dulaglutide
1.5mg was superior to comparator drugs in lowering HbA1c
in ve trials, and met the primary endpoint of non-inferiority
in the sixth. Further, in the three trials presented to date, we’ve
shown that the percent of patients achieving the American
Diabetes Association goal for HbA1c was signicantly greater
than the comparators. Patients taking dulaglutide 1.5mg also
showed weight loss for the duration of those trials.
In combination with our ready-to-use pen delivery
device, we believe once-a-week dulaglutide will be a very
competitive entry in the GLP-1 market.
In addition to empagliozin and dulaglutide, we have
two basal insulins in late-stage development.
In partnership with Boehringer Ingelheim, we submitted
our new insulin glargine product in the U.S., Europe, and Japan
in 2013. In addition, Lilly’s next-generation basal insulin, basal
insulin peglispro, is currently in Phase III trials. If the studies are
successful, we could submit basal insulin peglispro to regulatory
authorities as early as this year.
While we believe each of our potential new diabetes
medicines will oer important benets, it is our comprehen-
sive portfolio that gives Lilly a unique opportunity to help
people with diabetes meet their needs, while contributing
signicantly to Lilly’s return to growth post-2014.
Building on Lilly Leadership in Oncology
We also reached key milestones last year in oncology, an
important area of focus for our company. e unmet need is
huge: It’s estimated that, over a lifetime, cancer will strike one
of every two men and one of every three women in the United
States. We believe that, with our existing products Alimta® and
Erbitux®, our late-stage molecules ramucirumab and necitu-
mumab—both of which came from our acquisition of ImClone
in 2008—and our early- to mid-phase pipeline, we are well-
positioned to continue to be a leader in
oncology for many years to come.
Ramucirumab, which could launch
this year, has shown positive results in
two Phase III trials in patients with ad-
vanced gastric cancer. is is a devastat-
ing disease with no approved standard
of care in the U.S. or Europe.
In 2013, based on data from the
REGARD trial, we completed regulatory
submissions in the U.S. and Europe for
ramucirumab as a single-agent bio-
logic therapy in patients with advanced
gastric cancer. Based upon the results of
the RAINBOW trial, we also intend to
submit an application for ramucirumab
in combination with chemotherapy in
the rst half of 2014.
We recently announced that ramu-
cirumab improved overall survival in
patients with non-small cell lung cancer
(NSCLC) when combined with chemotherapy in a Phase III
trial. We intend to submit the rst regulatory application for
ramucirumab in NSCLC later this year.
In addition, we have ongoing Phase III ramucirumab
trials, expected to read out this year, in liver and colorectal
cancer. Depending on the trial data, we could submit the liver
cancer indication to regulators before the end of 2014. It’s
important to note that a Phase III study of ramucirumab in
breast cancer did not meet its primary endpoint.
Along with ramucirumab, we announced positive
Phase III results in 2013 for necitumumab, a fully-human
monoclonal antibody. Necitumumab demonstrated increased
overall survival as a rst-line treatment when patients with
stage IV metastatic squamous NSCLC were administered
necitumumab in combination with chemotherapy, versus
chemotherapy alone.
We believe that necitumumab represents an important
milestone for patients with squamous NSCLC—30 percent of
all NSCLC patients. is is a dicult-to-treat disease for which
there have been very limited advances in the last two decades.
We anticipate submitting necitumumab to regulatory
authorities before the end of 2014. If approved, necitumumab
would be the rst biologic agent approved to treat squamous
NSCLC—adding to Lilly’s leadership in lung cancer treatment.
(See page 10.)
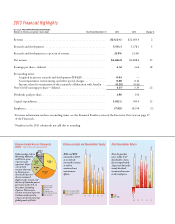
$232.6 +12%
$215.7 +9%
$160.6 +181%
$115.1 +6%
$108.7 +4%
$104.7 +142%
Cialis
Humalog
Trajenta
Animal Health
Alimta
Axiron
Key Contributors to 2013 Revenue Growth
($ in millions represent growth in revenue,
percent growth)
Five products and
a product line—
Cialis, Humalog,
Trajenta, Alimta,
Axiron, and
Animal Health—
together generated
revenue growth of
$937 million
during 2013 over
2012. is growth
was driven
primarily by
volume increases.