Eli Lilly 2013 Annual Report Download - page 13
Download and view the complete annual report
Please find page 13 of the 2013 Eli Lilly annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
11
Progress in treating lung cancer has been
incremental, but steady, amounting to an
additional nine months in mean overall
survival since the 1980s for patients with
the nonsquamous form of the disease. Lilly
scientists at our labs in Indianapolis, New
York, and San Diego continue to seek new
answers to this devastating disease.
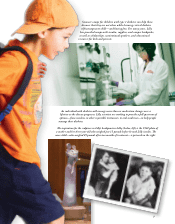
In a 1963 photo, a Lilly scientist demonstrates
that 15 tons of periwinkle plants were required to
make 1 ounce of Velban. To ensure a steady supply
of Velban, Lilly cultivated periwinkle plants on
massive farms in Texas.
Across tumor types, lung cancer—the
most prevalent cancer and biggest
killer—continues to be a key area of fo-
cus for Lilly. Our eorts in lung cancer
date from the ’90s with Gemzar, which
is approved for treatment of NSCLC as
well as other tumor types.
With Alimta, rst approved in 2004
for the treatment of mesothelioma,
we’ve changed the way NSCLC
is treated. Alimta was the rst
chemotherapy agent approved
specically for nonsquamous
NSCLC—and the rst to be approved
for maintenance treatment after initial
chemotherapy. Today, Alimta is the lead-
ing product for the treatment of rst-line
advanced nonsquamous NSCLC, with
shares ranging from nearly 40 percent to
over 70 percent in the countries around
the world.
Data from Phase III trials of necitu-
mumab—announced last year—and
ramucirumab—announced in early
2014—underscore Lilly’s continued
leadership in thoracic oncology.
Based on these results, we anticipate
submissions this year for review of
necitumumab as a rst-line treatment for
squamous NSCLC, and ramucirumab in
second-line NSCLC, including squamous
and nonsquamous histologies.
In the half-century since Lilly intro-
duced Velban, we’ve seen signicant
progress in cancer treatment, and today
we’re taking advantage of an explosion
of scientic knowledge to ght this
complex and tenacious foe.