Eli Lilly 2013 Annual Report Download
Download and view the complete annual report
Please find the complete 2013 Eli Lilly annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Determination Leads to Discovery
ELI LILLY AND COMPANY
2013 ANNUAL REPORT
NOTICE OF 2014 ANNUAL MEETING
PROXY STATEMENT
Table of contents
-
Page 1
Determination Leads to Discovery ELI LILLY AND COMPANY 2013 ANNUAL REPORT NOTICE OF 2014 ANNUAL MEETING PROXY STATEMENT -
Page 2
... to Discovery Pipeline of Molecules in Clinical Development Proxy Statement 1 2 6 14 16 17 19 19 20 23 36 37 38 47 48 52 54 55 57 59 60 Notice of Annual Meeting of Shareholders Proxy Statement Overview Board Operations and Governance Director Compensation Director Independence Committees of the... -
Page 3
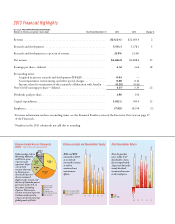
2013 Financial Highlights Eli lilly and Company and SubSidiariES (dollars in millions, except per-share data) year Ended december 31 2013 2012 Change % Revenue ...Research and development ...Research and development as a percent of revenue ...Net income ...Earnings per share-diluted ...Reconciling ... -
Page 4
...billion of operating cash flow, covering capital expenditures of $1 billion and allowing the company to return approximately $3.8 billion in cash to shareholders through the dividend and our share repurchase program. Creating an Unmatched Portfolio of Diabetes Medicines In 2013, Lilly took important... -
Page 5
... site in Suzhou to manufacture insulin for the Chinese market. In 2011, we established our China R&D head office in Shanghai, and the following year we opened the Lilly China Research and Development Center to focus specifically on type 2 diabetes in China. In addition, our Elanco animal health... -
Page 6
...our ready-to-use pen delivery Along with ramucirumab, we announced positive device, we believe once-a-week dulaglutide will be a very Phase III results in 2013 for necitumumab, a fully-human competitive entry in the GLP-1 market. monoclonal antibody. Necitumumab demonstrated increased In addition to... -
Page 7
... following surgery and helping me Lilly and our venture capital partners to facilitate early-stage recover and regain the full health that I enjoy today. development. The Capital Funds Portfolio is an outgrowth of The people of Eli Lilly and Company are proving that the FIPNet model we've pursued... -
Page 8
... companies were cutting R&D, Lilly stuck to our innovation-based strategy. Despite the financial pressures during the YZ period of patent expiries on a number of major products, we sustained investment in R&D and-as a result of that determined effort-we successfully rebuilt our late-stage pipeline... -
Page 9
"Research is the heart of the business, the soul of the enterprise." These are the words of Eli Lilly, president of Eli Lilly and Company, 1932-1948, and grandson of the founder. What "Mr. Eli" recognized in 1946 is still true today. The Wall Street Journal, October 21, 2013 7 -
Page 10
...24 hours a day to manage blood glucose levels between meals, including overnight. With Boehringer Ingelheim, we submitted our new insulin glargine product in the U.S., Europe, and Japan in 2013. Our new insulin glargine has been thoroughly studied in a clinical development program to ensure it meets... -
Page 11
... six medication changes over a lifetime as the disease progresses. Lilly scientists are working to provide a full spectrum of options-from insulins, to other injectable treatments, to oral medicines-to help people manage their diabetes. The inspiration for the sculpture in Lilly's headquarters lobby... -
Page 12
... non-small cell lung cancer (NSCLC). The global incidence of cancer will increase from an estimated 14 million new cases in 2012 to 22 million new cases a year within the next two decades, according to the World Health Organization. Lilly's ongoing clinical efforts seek to address tumors with high... -
Page 13
...taking advantage of an explosion of scientific knowledge to fight this complex and tenacious foe. Data from Phase III trials of necitumumab-announced last year-and ramucirumab-announced in early 2014-underscore Lilly's continued leadership in thoracic oncology. Progress in treating lung cancer has... -
Page 14
PIPELINE OF MOLECULES IN CLINICAL DEVELOPMENT REGULATORY REVIEW *Empagliflozin diabetes *new insulin glargine product diabetes ramucirumab solid tumors dulaglutide diabetes New Chemical Entity New Biotech Entity Diagnostic *Commercial collaboration PHASE III baricitinib rheumatoid arthritis ... -
Page 15
... standards promulgated by the Financial Accounting Standards Board and the Securities and Exchange Commission (SEC); • acquisitions and business development transactions; and • the impact of exchange rates and global macroeconomic conditions. Investors should not place undue reliance on forward... -
Page 16
... bring to market innovative new medicines. Our animal health business, operating through our Elanco division, develops, manufactures, and markets products for both food animals and companion animals. We manufacture and distribute our products through facilities in the United States, Puerto Rico, and... -
Page 17
... of pulmonary arterial hypertension. • • Cardiovascular products, including: • • • • Animal Health Products Our products for food animals include: • • • • Rumensin®, a cattle feed additive that improves feed efficiency and growth and also controls and prevents coccidiosis... -
Page 18
... business groups to service wholesalers, pharmacy benefit managers, managed-care organizations (MCOs), government and long-term care institutions, hospitals, and certain retail pharmacies. We enter into arrangements with these organizations providing for discounts or rebates on Lilly products. Human... -
Page 19
... the market or products that are later developed by competitors. If competitors introduce new products or delivery systems with therapeutic or cost advantages, our products can be subject to decreased sales, progressive price reductions, or both. We believe our long-term competitive success depends... -
Page 20
... by the U.S. Patent and Trademark Office. Patent term restoration is a statutory right provided to U.S. patents that claim inventions subject to review by the U.S. Food and Drug Administration (FDA). A single patent for a human pharmaceutical product may be eligible for patent term restoration to... -
Page 21
..., uses, formulations, or processes do not preclude other manufacturers from employing alternative processes or marketing alternative products or formulations that compete with our patented products. In addition, competitors or other third parties sometimes may assert claims that our activities... -
Page 22
... mark is used, and in other countries as long as it is registered. Registrations are normally for fixed but renewable terms. Patent Licenses Most of our major products were discovered in our own laboratories and are not subject to significant license agreements. Two of our largest products, Cialis... -
Page 23
..., state, and local agencies. The lengthy process of laboratory and clinical testing, data analysis, manufacturing development, and regulatory review necessary for governmental approvals is extremely costly and can significantly delay product introductions. Promotion, marketing, manufacturing, and... -
Page 24
...failure to supply product to us or delays in new product approvals. The marketing, promotional, and pricing practices of human pharmaceutical manufacturers, as well as the manner in which manufacturers interact with purchasers and prescribers, are subject to various other U.S. federal and state laws... -
Page 25
..., including licensing arrangements, co-development and co-marketing agreements, copromotion arrangements, joint ventures, and acquisitions. Our Elanco animal health innovation strategy is focused on identifying and developing promising technologies and potential products from internal and external... -
Page 26
... 60 drug candidates across all stages of human testing and a larger number of projects in preclinical development. Among our new investigational molecules currently in the product phase of development or awaiting regulatory approval are potential therapies for diabetes, various cancers, Alzheimer... -
Page 27
... United Kingdom. Finishing operations, including formulation, filling, assembling, delivery device manufacturing, and packaging, take place at a number of sites throughout the world. We utilize third parties for certain active ingredient manufacturing and finishing operations. We manage our supply... -
Page 28
... in the short-term or long-term would have a material adverse effect on our business, results of operations, cash flows, financial position and prospects. • We face intense competition from multinational pharmaceutical companies, biotechnology companies, and lower-cost generic manufacturers. We... -
Page 29
...: Compound patent 2015 For non-biological products, loss of exclusivity (whether by expiration or as a consequence of litigation) typically results in the entry of one or more generic competitors, leading to a rapid and severe decline in revenues. For biological products (such as Humalog, Humulin... -
Page 30
... of new products pending resolution of the issues. We are now operating under a Corporate Integrity Agreement with the Office of Inspector General of the U.S. Department of Health and Human Services that requires us to maintain comprehensive compliance programs governing our research, manufacturing... -
Page 31
... malicious intrusions by private or governmental actors through human or electronic means, including "hacking" or "cyber-attacks," or through negligent or wrongful conduct by employees or others with permitted access to our systems and data. The rapid growth of social media exacerbates the risk... -
Page 32
... the research and development, acquisition, and licensing efforts to generate new products. The failure to manage these risks could have a material adverse effect on our revenues. • Worsening economic conditions could adversely affect our business and operating results. While human pharmaceuticals... -
Page 33
... by higher research and development expenses and lower other income. EPS in 2013 also benefited from a lower number of shares outstanding compared to 2012 as a result of our share repurchase programs. The following highlighted items affect comparisons of our 2013 and 2012 financial results: 2013... -
Page 34
...products and acquire or collaborate on molecules currently in development by other biotechnology or pharmaceutical companies. We currently have approximately 60 potential new drugs in human testing or under regulatory review, and a larger number of projects in preclinical research. The following new... -
Page 35
...that the marketing authorization application for our new insulin glargine product, filed in June 2013 through the biosimilar pathway, was accepted for review by the European Medicines Agency. In the fourth quarter of 2013, we filed for regulatory review in the U.S. and Japan. In January 2014, Sanofi... -
Page 36
... of our revenues, cash flows, and earnings. Cymbalta® lost patent exclusivity in the U.S. in December 2013, resulting in the immediate entry of several generic competitors. We also expect the loss of U.S. patent protection for Evista® in March 2014 to result in immediate generic competition. We... -
Page 37
... changes to key elements of the global international tax framework could have a material adverse effect on our consolidated results of operations. Operating Results-2013 Revenue Our worldwide revenue for 2013 increased 2 percent, to $23.11 billion, compared with 2012 as an increase of 5 percent due... -
Page 38
...includes revenue in Puerto Rico. Collaboration and other revenue in 2013 consists primarily of royalties for Erbitux® and revenue associated with Trajenta. Collaboration and other revenue in 2012 also includes revenue associated with exenatide in the United States. Sales of Cymbalta, a product for... -
Page 39
... in U.S. sales and marketing activities in anticipation of the loss of patent exclusivity for Cymbalta and Evista, as well as the impact of foreign exchange rates. Research and development expenses increased 5 percent to $5.53 billion in 2013, due to higher research and clinical development expenses... -
Page 40
...-time impact of the reinstatement of the R&D tax credit for 2012 that was recorded in the first quarter of 2013. See Note 14 to the consolidated financial statements for additional information. Operating Results-2012 Financial Results Worldwide total revenue decreased 7 percent to $22.60 billion in... -
Page 41
...Cialis ...360.4 Zyprexa ...592.1 Humulin ...488.2 Forteo ...699.5 Evista ...384.1 Strattera ...339.0 Effient...593.4 Other pharmaceutical products ...1,161.8 Animal health products ...Total net product sales...11,811.8 501.3 Collaboration and other revenue(2) ...Total revenue...$ 12,313.1 1 2 1,076... -
Page 42
... statements for additional information. FINANCIAL CONDITION As of December 31, 2013, cash and cash equivalents decreased to $3.83 billion compared with $4.02 billion at December 31, 2012, as cash flow from operations of $5.74 billion was more than offset by dividends paid of $2.12 billion, share... -
Page 43
... backs our commercial paper program. Capital expenditures of $1.01 billion during 2013 were $106.7 million more than in 2012. We expect 2014 capital expenditures to be approximately $1.3 billion as we invest in the long-term growth of our diabetes-care product portfolio and additional biotechnology... -
Page 44
...effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures, or capital resources. We acquire and collaborate on potential products still in development and enter into research and development arrangements with third... -
Page 45
... of operations, financial position, or liquidity for the periods presented in this report. Our most critical accounting estimates have been discussed with our audit committee and are described below. Revenue Recognition and Sales Return, Rebate, and Discount Accruals We recognize revenue from sales... -
Page 46
..., long-term care, hospital, patient assistance programs, and various other programs. We base these accruals primarily upon our historical rebate and discount payments made to our customer segment groups and the provisions of current rebate and discount contracts. The largest of our sales rebate... -
Page 47
... reported. In addition to the analysis below, see Note 15 to the consolidated financial statements for additional information regarding our retirement benefits. Annually, we evaluate the discount rate and the expected return on plan assets in our defined benefit pension and retiree health benefit... -
Page 48
...the 2013 service cost and interest cost by $7.6 million. If the 2013 discount rate for the U.S. defined benefit pension and retiree health benefit plans (U.S. plans) were to change by a quarter percentage point, income before income taxes would change by $40.6 million. If the 2013 expected return on... -
Page 49
... business development transactions. Operating cash flows are expected to be sufficient to pay our dividend of approximately $2.1 billion, allow for capital expenditures of approximately $1.3 billion, and fund potential business development activity and share repurchases. Our 2014 financial guidance... -
Page 50
Financial Statements and Supplementary Data Consolidated Statements of Operations ELI LILLY AND COMPANY AND SUBSIDIARIES (Dollars in millions, except per-share data) Year Ended December 31 2013 2012 2011 Revenue ...$ 23,113.1 Cost of sales ...4,908.1 Research and development ...5,531.3 Marketing, ... -
Page 51
...204.3 88.5 (178.5) Defined benefit pension and retiree health benefit plans (Note 15) . . 2,592.2 (128.6) (1,240.2) Effective portion of cash flow hedges ...(123.8) 8.7 44.8 Other comprehensive income (loss) before income taxes ...2,708.9 129.5 (1,618.7) Provision for income taxes related to other... -
Page 52
...) ...Accrued retirement benefits (Note 15) ...Long-term income taxes payable (Note 14) ...Other noncurrent liabilities ...Total other liabilities ...Commitments and contingencies (Note 16) Eli Lilly and Company Shareholders' Equity (Notes 11 and 12) Common stock-no par value Authorized shares: 3,200... -
Page 53
... 31, 2012 . Net income ...Other comprehensive income (loss), net of tax ...Cash dividends declared per share: $1.96 ...Retirement of treasury shares . . Purchase for treasury ...Issuance of stock under employee stock plans-net ...Stock-based compensation ...Balance at December 31, 2013 . 1 1,154... -
Page 54
... short-term investments ...Purchases of short-term investments ...Proceeds from sales and maturities of noncurrent investments ...Purchases of noncurrent investments ...Purchase of product rights ...Purchases of in-process research and development ...Cash paid for acquisitions, net of cash acquired... -
Page 55
... dates of less than one year from the date of the balance sheet are classified as short-term. Available-for-sale securities are carried at fair value with the unrealized gains and losses, net of tax, reported in other comprehensive income (loss). The credit portion of unrealized losses on our debt... -
Page 56
... development and commercialization, to the estimated future net cash flows that are derived from projected sales revenues and estimated costs. These projections are based on factors such as relevant market size, patent protection, historical pricing of similar products, and expected industry trends... -
Page 57
... approval for marketing or launch of the product), we amortize the payment to income as we perform under the terms of the arrangement. See Note 4 for specific agreement details. Royalty revenue from licensees, which is based on third-party sales of licensed products and technology, is recorded... -
Page 58
... tax benefit should be presented in the financial statements as a separate liability. The assessment is based on the unrecognized tax benefit and deferred tax asset that exist at the reporting date. The provisions of the new standard are effective on a prospective basis beginning in 2014 for annual... -
Page 59
...and royalties based on sales should these products be approved for commercialization. Acquisition of Businesses ChemGen On February 17, 2012, we acquired all of the outstanding stock of ChemGen Corporation, a privately-held bioscience company specializing in the development and commercialization of... -
Page 60
... the terms of our prior arrangement, we reported as collaboration and other revenue our 50 percent share of gross margin on Amylin's net product sales in the United States. We reported as net product sales 100 percent of sales outside the U.S. and our sales of Byetta pen delivery devices to Amylin... -
Page 61
... received by us for supply of clinical trial materials; for research and development; and for a portion of marketing, selling, and administrative expenses are recorded as a reduction to the respective expense line items on the consolidated statement of operations. We receive a distribution fee... -
Page 62
... the new insulin glargine product are both under regulatory review in the U.S., Europe, and Japan, and the anti-TGF-beta monoclonal antibody is in Phase II clinical testing. In connection with the approval of linagliptin in the U.S., Japan, and Europe, in 2011 we paid $478.7 million in success-based... -
Page 63
... royalty payments on future global sales with rates ranging up to 20 percent if the product is successfully commercialized. The agreement provides Incyte with options to co-develop these compounds on an indication-by-indication basis by funding 30 percent of the associated development costs from the... -
Page 64
... of total inventories at December 31, 2013 and 2012, respectively. Note 7: Financial Instruments Financial instruments that potentially subject us to credit risk consist principally of trade receivables and interest-bearing investments. Wholesale distributors of life-sciences products account for... -
Page 65
... Instruments on the Statement of Operations The following effects of risk-management instruments were recognized in other-net, (income) expense: 2013 2012 2011 Fair value hedges: Effect from hedged fixed-rate debt ...$ (308.2) Effect from interest rate contracts ...308.2 Cash flow hedges: Effective... -
Page 66
... (Level 3) Description Carrying Amount Amortized Cost Fair Value December 31, 2013 Cash and cash equivalents ...$ 3,830.2 Short-term investments: U.S. government and agencies ...$ 276.4 Corporate debt securities ...931.7 Other securities ...2.7 Marketable equity ...356.3 Short-term investments... -
Page 67
... the risk-management instruments above, individually, these financial rights are not material. We determine fair values based on a market approach using quoted market values, significant other observable inputs for identical or comparable assets or liabilities, or discounted cash flow analyses. The... -
Page 68
... and other indefinite-lived intangible assets at December 31 were as follows: 2013 2012 Goodwill (by segment): Human pharmaceutical products ...$ Animal health ...Total goodwill ...In-process research and development ...Total indefinite-lived intangible assets...$ 1,354.7 162.1 1,516.8 33.6 1,550... -
Page 69
... global jurisdiction (U.S., Europe, and Japan) and capitalized milestone payments. Other intangibles consist primarily of the amortized cost of licensed platform technologies that have alternative future uses in research and development, manufacturing technologies, and customer relationships... -
Page 70
...equipment, net on the consolidated balance sheets, capital lease obligations entered into, and future minimum rental commitments are not material. Note 10: Borrowings Long-term debt at December 31 consisted of the following: 2013 2012 4.20 to 7.13 percent notes (due 2014-2037) ...$ Other, including... -
Page 71
... of cash flows. At December 31, 2013, additional stock-based compensation awards may be granted under the 2002 Lilly Stock Plan for not more than 100.0 million shares. Performance Award Program PAs are granted to officers and management and are payable in shares of our common stock. The number of... -
Page 72
... December 31, 2013 and 2012, no preferred stock has been issued. We have an employee benefit trust that held 50.0 million shares of our common stock at both December 31, 2013 and 2012, to provide a source of funds to assist us in meeting our obligations under various employee benefit plans. The cost... -
Page 73
... to purchase shares of our common stock on the open market. As of December 31, 2013, all shares of common stock held by the ESOP were allocated to participating employees as part of our savings plan contribution. The fair value of shares allocated each period was recognized as compensation expense... -
Page 74
... follows: 2013 2012 Deferred tax assets: Compensation and benefits ...$ Tax credit carryforwards and carrybacks ...Purchases of intangible assets ...Product return reserves ...Tax loss carryforwards and carrybacks ...Debt ...Contingencies ...Intercompany profit in inventories ...Sale of intangibles... -
Page 75
... rate to income before income taxes to reported income tax expense: 2013 2012 2011 Income tax at the U.S. federal statutory tax rate...$ Add (deduct): International operations, including Puerto Rico ...General business credits ...IRS audit conclusion ...Other ...Income taxes ...$ 2,061.3 $ 1,892... -
Page 76
... to develop the change in benefit obligation, change in plan assets, funded status, and amounts recognized in the consolidated balance sheets at December 31 for our defined benefit pension and retiree health benefit plans, which were as follows: Defined Benefit Pension Plans 2013 2012 Retiree Health... -
Page 77
... Benefit Pension Plans 2013 2012 2011 Retiree Health Benefit Plans 2013 2012 2011 (Percents) Discount rate for benefit obligation ...Discount rate for net benefit costs ...Rate of compensation increase for benefit obligation ...Rate of compensation increase for net benefit costs ...Expected return... -
Page 78
... global benefit plans may enter into contractual arrangements (derivatives) to implement the local investment policy or manage particular portfolio risks. Derivatives are principally used to increase or decrease exposure to a particular public equity, fixed income, commodity, or currency market more... -
Page 79
... investments include venture capital (early stage investing), buyout, and special situation investing. Private equity management firms typically acquire and then reorganize private companies to create increased long term value. Private equity-like funds usually have a limited life of approximately... -
Page 80
... (Level 3) Asset Class Total Defined Benefit Pension Plans Public equity securities: U.S...$ International ...Fixed income: Developed markets ...Emerging markets ...Private alternative investments: Hedge funds ...Equity-like funds ...Real estate ...Other ...Total ...$ Retiree Health Benefit Plans... -
Page 81
The activity in the Level 3 investments during the year ended December 31, 2013 was as follows: Fixed Income: Developed Markets Hedge Funds Equity-like Funds Real Estate Total Defined Benefit Pension Plans Beginning balance at January 1, 2013 ...$ Actual return on plan assets, including changes... -
Page 82
... Total ...$ 8,286.6 Retiree Health Benefit Plans Public equity securities: U.S...$ 45.4 International ...127.7 Fixed income: Developed markets ...59.4 Emerging markets ...40.3 Private alternative investments: Hedge funds ...234.0 Equity-like funds ...81.9 Cash value of trust owned insurance contract... -
Page 83
The activity in the Level 3 investments during the year ended December 31, 2012 was as follows: Fixed Income: Developed Markets Hedge Funds Equity-like Funds Real Estate Total Defined Benefit Pension Plans Beginning balance at January 1, 2012 ...$ Actual return on plan assets, including changes... -
Page 84
... litigation. In June 2013, Accord filed a petition requesting review of the patent by the U.S. Patent and Trademark Office, which was denied in October 2013. This denial is final and cannot be appealed. Generic manufacturers have filed an opposition to the European Patent Office's decision to grant... -
Page 85
... related to our defined benefit pension and retiree health benefit plans (Note 15) was an expense of $886.1 million in 2013, an expense of $34.4 million in 2012, and a benefit of $383.8 million in 2011. The tax effect on the effective portion of cash flow hedges was a benefit of $43.2 million for... -
Page 86
... from the transfer to Amylin of exenatide commercial rights in all markets outside the U.S. in 2013 and income recognized from the early payment of the exenatide revenue-sharing obligation by Amylin in 2012. See Note 4 for additional information. For the year ended December 31, 2011, other-net... -
Page 87
... of consolidated total revenue. Further, they each accounted for between 9 percent and 18 percent of accounts receivable as of December 31, 2013 and 2012. Animal health products are sold primarily to wholesale distributors. We manage our assets on a total company basis, not by operating segment, as... -
Page 88
... ended December 31, 2013, 2012, and 2011, respectively. For internal management reporting presented to the chief operating decision maker, certain costs are fully allocated to our human pharmaceutical products segment and therefore are not reflected in the animal health segment's profit. Such items... -
Page 89
...of sales ...1,248.3 Operating expenses(1) ...3,440.6 Asset impairment, restructuring, and other special charges . 204.0 Other-net, (income) expense ...52.0 Income before income taxes ...1,012.4 Net income ...827.2 Earnings per share-basic ...0.75 Earnings per share-diluted ...0.74 Dividends paid per... -
Page 90
... system of internal controls, and our people, who are objective in their responsibilities and operate under a code of conduct and the highest level of ethical standards. Management's Report on Internal Control Over Financial Reporting-Eli Lilly and Company and Subsidiaries Management of Eli Lilly... -
Page 91
... 31, 2013. Their responsibility is to evaluate whether internal control over financial reporting was designed and operating effectively. John C. Lechleiter, Ph.D. Chairman, President, and Chief Executive Officer February 19, 2014 Derica W. Rice Executive Vice President, Global Services and Chief... -
Page 92
... and Shareholders of Eli Lilly and Company We have audited the accompanying consolidated balance sheets of Eli Lilly and Company and subsidiaries as of December 31, 2013 and 2012, and the related consolidated statements of operations, comprehensive income, shareholders' equity, and cash flows for... -
Page 93
... Registered Public Accounting Firm The Board of Directors and Shareholders of Eli Lilly and Company We have audited Eli Lilly and Company and subsidiaries' internal control over financial reporting as of December 31, 2013, based on criteria established in Internal Control-Integrated Framework... -
Page 94
...) ELI LILLY AND COMPANY AND SUBSIDIARIES (Dollars in millions, except revenue per employee and per-share data) 2013 2012 2011 2010 2009 Operations Revenue ...$ 23,113.1 $ 22,603.4 $ 24,286.5 $ 23,076.0 $ 21,836.0 Cost of sales ...4,908.1 4,796.5 5,067.9 4,366.2 4,247.0 Research and development... -
Page 95
... stock, the S&P 500 Stock Index, and the peer groups' common stock. The graph measures total shareholder return, which takes into account both stock price and dividends. It assumes that dividends paid by a company are reinvested in that company's stock. Value of $100 Invested on Last Business Day... -
Page 96
... Used In This Report Trademarks or service marks owned by Eli Lilly and Company or its subsidiaries or affiliates, when first used in this report, appear with an initial capital and are followed by the symbol ® or ™, as applicable. In subsequent uses of the marks in the report, the symbols may... -
Page 97
Notice of 2014 Annual Meeting of Shareholders and Proxy Statement Your vote is important Please vote by using the Internet, telephone, or by signing, dating, and returning the enclosed proxy card. -
Page 98
... of Annual Meeting of Shareholders 2013 - Proxy Statement Overview Board Operations and Governance Director Compensation Director Independence Committees of the Board of Directors Membership and Meetings of the Board and Its Committees Board Oversight of Compliance and Risk Management Highlights... -
Page 99
... 2014 Annual Meeting of Shareholders of Eli Lilly and Company will be held as shown below: WHEN: WHERE: 11:00 a.m. EDT, Monday, May 5, 2014 The Lilly Center Auditorium Lilly Corporate Center Indianapolis, Indiana 46285 ITEMS OF BUSINESS: Election of the five directors listed in the proxy statement... -
Page 100
... read the entire proxy statement carefully before voting. Meeting: Time: Record Date: Annual Meeting of Shareholders 11:00 a.m. EDT February 28, 2014 Date: Location: May 5, 2014 The Lilly Center Auditorium Lilly Corporate Center Indianapolis, Indiana 46285 What Is New In This Year's Proxy Below is... -
Page 101
... majority of the CEO's pay long-term focused and linked to company performance and shareholder value, the Compensation Committee only increased Dr. Lechleiter's target equity compensation. Dr. Lechleiter's base salary and annual bonus targets remained unchanged. The named executive officers... -
Page 102
...at every regularly-scheduled board meeting Active board participation in company strategy and CEO succession planning Board oversight of compliance and enterprise risk management practices Meaningful director stock ownership guidelines Majority voting standard and resignation policy for the election... -
Page 103
... pay to performance through a blend of short- and long-term performance measures The Compensation Committee annually reviews compensation programs to ensure appropriate risk mitigation No "top hat" retirement plans - supplemental plans are open to all employees and are limited to restoring benefits... -
Page 104
... of the ability or integrity of any of our directors or nominees for director during the past 10 years. Class of 2014 The following six directors' terms will expire at this year's annual meeting. Dr. Gilman will retire from the Board at the end of his term. The other five directors are standing for... -
Page 105
... Board Committees: Compensation (chair); Directors and Corporate Governance Career Highlights Other Board Service Brock Capital Group, a provider of financial advising and • Public boards: T. Rowe Price Mutual consulting services Funds; Simon Property Group, Inc.; and • Senior Managing Director... -
Page 106
... Indianapolis, the National University • Non-profit boards: United Way Worldwide; Xavier of Ireland, and Indiana University University; the Life Sciences Foundation; and the Central Indiana Corporate Partnership Qualifications: Dr. Lechleiter is our chairman, president, and chief executive officer... -
Page 107
... Prior public board service: Cadbury plc Non-profit boards: Wellesley College; Tropicana Beverage Group - Pepsico Institute for the Future; New York-Presbyterian President and Chief Executive Officer (1993 - 1998 Hospital; Lincoln Center Theater; and Families Nabisco Biscuit Company, a unit of... -
Page 108
... search firm. Board Committees: Audit; Finance Career Highlights Other Board Service DBS Group Holdings and DBS Bank (formerly the • Public boards: The Bank of China Limited, Development Bank of Singapore), one of the largest Singapore Airlines, MasterCard financial services groups in Asia... -
Page 109
... 5, 2014, prior to the annual meeting of shareholders, and the Directors and Corporate Governance Committee does not plan to fill his vacant seat. Ralph Alvarez, age 58, director since 2009 Board Committees: Compensation; Science and Technology Career Highlights Other Board Service • Public boards... -
Page 110
... customers • Non-profit companies: Boulder • • Community Hospital; Children's Hospital • Chairman (2002 2013) • Colorado • • President and Chief Executive Officer (2001 - 2010 Chief Operating Officer (2000 - 2001) • Prior public board service: Irwin Financial • • Corporation... -
Page 111
... wishes to recommend a director candidate for evaluation should forward the candidates name and information about the candidate's qualifications to: Chair of the Corporate Governance Committee c/o Corporate Secretary Lilly Corporate Center Indianapolis, IN 46285 The candidate must meet the selection... -
Page 112
... times their annual cash retainer; new directors are allowed five years to reach this ownership level. Nonemployee directors receive $145,000 of stock compensation, deposited annually in a deferred stock account in the Lilly Directors' Deferral Plan (as described below), payable after service on the... -
Page 113
...in a lump sum or in annual installments for up to 10 years, beginning the second January following the director's departure from board service. Amounts in the deferred stock account are paid in shares of company stock. 2013 Director Compensation Fees Earned or Paid in Cash ($) $106,000 $103,000 $112... -
Page 114
... column consists of amounts donated by the Eli Lilly and Company Foundation, Inc. ("Foundation") under its matching gift program, which is generally available to U.S. employees as well as the outside directors. Under this program, the Foundation matched 100 percent of charitable donations over $25... -
Page 115
... its own charter annually, and the Directors and Corporate Governance Committee reviews and approves all committee charters annually. The chair of each committee determines the frequency and agenda of committee meetings. The Audit, Compensation, and Public Policy and Compliance Committees meet alone... -
Page 116
... and strategies; Dividends; Stock repurchases; Capital expenditures; Investments, financings and borrowings; Financial risk management; and Significant business-development projects. Public Policy and Compliance Committee • • Oversees the processes by which the company conducts its business so... -
Page 117
... operates with the highest level of integrity and that the company is appropriately managing both current and potential future areas of risk. Code of Ethics The board approves the company's code of ethics, which is set out in: The Red Book: a comprehensive code of ethical and legal business conduct... -
Page 118
...unit reviews and at the annual board and senior management strategy session. Highlights of the Company's Corporate Governance The company is committed to good corporate governance, which promotes the long-term interest of shareholders and other company stakeholders, builds confidence in our company... -
Page 119
... by major shareholders, ensures that she is available for consultation and direct communication; Approving meeting agendas and schedules and generally approving information sent to the Board; Conducting executive sessions of the independent directors; and Overseeing the independent directors' annual... -
Page 120
... sessions for the Audit Committee. Additionally, the Directors and Corporate Governance Committee conducts an annual assessment of the Board's performance, Board committee performance, and all Board processes based on input from all directors. Prior Management Proposals to Eliminate Classified... -
Page 121
... persons (directors and executive officers, their immediate family members, or shareholders of 5 percent or greater of the company's outstanding stock). The policy covers any relatedperson transaction that meets the minimum threshold for disclosure in the proxy statement under the relevant SEC rules... -
Page 122
... are committed to the company's core values of integrity, excellence, and respect for people. Our compensation programs are designed to help us achieve these goals while balancing the long-term interests of our customers and shareholders. Objectives Our compensation and benefits program is based on... -
Page 123
... with Lilly, have similar business models, and seek to hire from the same pool management and scientific talent. In the aggregate, the company's total hire from the same pool of management and scientific talent. In the aggregate, the company's total compensation to named executive officers for 2012... -
Page 124
.... In setting salaries, the Compensation Committee seeks to retain, motivate, and reward successful performers while maintaining affordability within the company's business plan. 2. Annual Bonus The Eli Lilly and Company Bonus Plan ("Bonus Plan") is designed to align employees' individual goals... -
Page 125
...performance period. SVAs pay out above target if Lilly stock outperforms an expected compounded annual rate of return and below target if company stock underperforms that rate of return. The expected rate of return includes dividends and is based on the total three-year shareholder return (TSR) that... -
Page 126
... advance the product pipeline, with 11 molecules in late-stage development. The directors also noted Dr. Lechleiter's strong leadership in establishing and executing the company's strategy to manage through the period of patent expirations from 2011-2014 and return to long-term growth following this... -
Page 127
... on internal pay relativity and market data. Name Dr. Lechleiter Mr. Rice Dr. Lundberg Mr. Harrington Mr. Conterno 2013 140% 90% 90% 75% 75% The Compensation Committee established the performance targets for 2013 equal to the targets specified in the company's 2013 corporate operating plan. 29 -
Page 128
....64 Greater than 9% 140% Compounded Annual Share Price Growth Less than (4.2%) Rate (excluding dividends) Percent of Target 0% 2013 Compensation Payouts The information in this section reflects the amounts paid to NEOs for the 2013 annual bonus and payouts from equity awards for which the relevant... -
Page 129
...in the "Executive Compensation" section of the proxy that follows. Equity Award Payouts in 2013 2012-2013 Performance Award The target cumulative EPS for the 2012-2013 PA was set in January of 2012 reflecting expected industry growth of 3.3 percent each year. The company's two-year EPS growth was at... -
Page 130
... Annual Growth â- â- Target Payout Actual Payout For the NEOs, the number of shares paid out under the 2012-2013 PA is reflected in the table below (this information is also included in footnote 5 to the "Outstanding Equity Awards Table" in the "Executive Compensation" section of the proxy... -
Page 131
...replaced. Employee Benefits The company offers core employee benefits coverage to provide our workforce with a reasonable level of financial support in the event of illness or injury provide post-retirement income; and enhance productivity and job satisfaction through benefit programs that focus... -
Page 132
... benefits received by the employee in connection with a change in control could exceed limits established under Section 280G of the Internal Revenue Code. The employee would then be subject to an excise tax on top of normal federal income tax. The company does not reimburse employees for these taxes... -
Page 133
... and for 2013, executive officers did not hold any pledged shares. Effective in 2014, the committee adopted a formal policy prohibiting outside directors and all members of senior management from pledging any company stock. Tax Deductibility Cap on Executive Compensation U.S. federal income tax law... -
Page 134
... nor her firm is permitted to have any business or personal relationship with management or the members of the Compensation Committee. The consultant's responsibilities are to: • Review the company's total compensation philosophy, peer group, and target competitive positioning for reasonableness... -
Page 135
... Committee has downward discretion to lower compensation plan payouts Threshold levels below target that provide for payouts and maximums that cap payouts Different measures and metrics used across multiple incentive plans; appropriate balance of cash/stock, fixed/variable pay, short-term/long-term... -
Page 136
...875 $3,718,214 Derica W. Rice Executive Vice President, Global Services and Chief Financial Officer Jan M. Lundberg, Ph.D. Executive Vice President, Science and Technology and President, Lilly Research Laboratories Michael J. Harrington 2013 2012 2011 2013 2012 2011 2013 Senior Vice President and... -
Page 137
... Awards: Number of Shares of Stock, Options, or Units Name Award Grant Date 2 Compensation Committee Action Date Threshold ($) Target ($) Maximum ($) Threshold (# shares) Target (# shares) Maximum (# shares) Grant Date Fair Value of Equity Awards Dr. Lechleiter 2013-2014 PA 2013-2015 SVA... -
Page 138
... Expiration Date Award 2013-2015 SVA 2012-2014 SVA 2013-2014 PA 2012-2013 PA 2011-2012 PA Number of Shares or Units of Stock That Have Not Vested (#) Market Value of Shares or Units of Stock That Have Not Vested ($) Equity Incentive Plan Awards: Number of Unearned Shares, Units, or Other Rights... -
Page 139
... from any payout must be held by executive officers for a minimum of one year. Had the performance period ended December 31, 2013, the payout would have been 60 percent of target. SVAs granted for the 2012-2014 performance period. The number of shares reported in the table reflects the 40 maximum... -
Page 140
... named executive officers. • The retirement plan, a tax-qualified defined benefit plan that provides monthly benefits to retirees. See the "Pension Benefits in 2013" table below for additional information about the value of these pension benefits. Sections 401 and 415 of the Internal Revenue Code... -
Page 141
... the company longer. For the transition group, early retirement benefits are reduced 3 percent for each year from age 65 to age 60 and 6 percent for each year under age 60. All named executive officers except Dr. Lundberg are in this transition group. Pre-2010 Plan Information: Employees hired prior... -
Page 142
...(d) of the Internal Revenue Code with monthly compounding, which was 2.9 percent for 2013 and is 3.9 percent for 2014. Participants may elect to receive the funds in a lump sum or in up to 10 annual installments following retirement, but may not make withdrawals during their employment, except in... -
Page 143
...Severance Pay Plan-Continuation of medical and welfare benefits" below. Equity grants include an individual performance criterion to vest. As a result, even retirement-eligible employees have the possibility of forfeiting their grants. The company eliminated excise tax gross-ups in 2012. Accrued Pay... -
Page 144
...named executive officer's compensation, benefits, age, and service the terms of based on the executive officer's compensation, benefits, age, and the terms of the the plan, plan, based on the named named executive officer's compensation, benefits, age, and service service credit at December 31, 2013... -
Page 145
... The following table sets forth the number of shares of company common stock beneficially owned by the directors, the named executive officers, and all directors and executive officers as a group, as of February 21, 2014. None of the stock, stock options, or stock units owned by any of the listed... -
Page 146
... shares of the company's common stock, as of December 31, 2013, are the shareholders listed below: Name and Address Lilly Endowment, Inc. (the Endowment) 2801 North Meridian Street Indianapolis, Indiana 46208 BlackRock, Inc. 40 East 52nd Street New York, New York 10022 Wellington Management Company... -
Page 147
... Independent Auditor Audit Committee Report The Audit Committee reviews the company's financial reporting process on behalf of the Board. Management has the primary responsibility for the financial statements and the reporting process, including the systems of internal controls and disclosure... -
Page 148
...'s policy and procedures are as follows: • The committee approves the annual audit services engagement and, if necessary, any changes in terms, conditions, and fees resulting from changes in audit scope, company structure, or other matters. Audit services include internal controls attestation work... -
Page 149
... performance of the audit or reviews of the financial statements - 2013 and 2012: primarily related to employee benefit plan and other ancillary audits, and due diligence services on potential acquisitions Tax Fees • 2013 and 2012: primarily related to consulting and compliance services All Other... -
Page 150
...motivate them to create long-term shareholder value by achieving top-tier corporate performance while embracing the company's values of integrity, excellence, and respect for people. 51 The Compensation Committee and the Board of Directors believe that our executive compensation aligns well with our... -
Page 151
... for a small number of shares from a prior stock ownership plan, which can be voted only on the directions of the participants to whose accounts the shares are credited). All participants are named fiduciaries under the terms of the 401(k) plan and under the Employee Retirement Income Security Act... -
Page 152
... November 24, 2014. Proposals should be addressed to the company's corporate secretary, Lilly Corporate Center, Indianapolis, Indiana 46285. In addition, the company's bylaws provide that any shareholder wishing to propose any other business at the annual meeting must give the company written notice... -
Page 153
...ownership reporting compliance Under SEC rules, our directors and executive officers are required to file with the SEC reports of holdings and changes in beneficial ownership of company stock. We have reviewed copies of reports provided to the company, as well as other records and information. Based... -
Page 154
... for 2012-2013 PA. When the Compensation Committee set EPS growth goals for the 2012-2013 PA, the termination of our exenatide alliance with Amylin and the associated revenue-sharing obligation was not contemplated and therefore, the 2012-2013 PA goals assumed ongoing net income from sales of... -
Page 155
Annual Meeting Admission Ticket Eli lilly and Company 2014 annual meeting of Shareholders monday, may 5, 2014 11:00 a.m. EdT lilly Center auditorium lilly Corporate Center indianapolis, indiana 46285 The top portion of this page will be required for admission to the meeting. Please write your name ... -
Page 156
... of this page with you to the meeting. Detach here Detach here Eli Lilly and Company Annual Meeting of Shareholders May 5, 2014 Complimentary parking lilly Corporate Center Please place this identifier on the dashboard of your car as you enter Lilly Corporate Center so it can be clearly seen by... -
Page 157
..., Development, and Delivery Andrew Hotchkiss President, Europe/Australia/Canada Operations Myles O'Neill Senior Vice President, Global Parenteral Drug Product and Delivery Devices Manufacturing Joshua L. Smiley Senior Vice President, Finance, and Chief Financial Officer, Lilly Research Laboratories... -
Page 158
... The annual meeting of shareholders will be held at the Lilly Center Auditorium, Lilly Corporate Center, Indianapolis, Indiana, on Monday, May 5, 2014, at 11:00 a.m. EDT. For more information, see the proxy statement section of this report. 10-K and 10-Q reports Paper copies of the company's annual... -
Page 159
... of our business in our 2012-13 Corporate Responsibility Report at www.lilly.com/responsibility/our-approach. As part of an assignment with the Lilly NCD Partnership, Lilly Connecting Hearts Abroad ambassadors Sandra James (seated, on left) and Taylor Burch (standing, on right) helped train workers... -
Page 160
Eli Lilly and Company Lilly Corporate Center Indianapolis, Indiana 46285 USA 317-276-2000 www.lilly.com