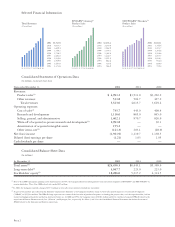
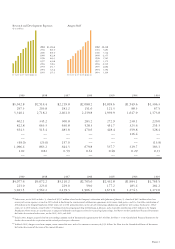
Amgen 2002 Annual Report Download - page 12
Download and view the complete annual report
Please find page 12 of the 2002 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Page 10
board on our other financial, operational, scientific,
and medical priorities. By any measure it was a very
productive year for us.
However, the year for corporate America was in gen-
eral troubling. Corporate governance was in the news
and the incidents were often deeply disturbing. All
good companies looked hard at their own businesses
to be sure that the procedures, environment, and
approach to corporate governance were right and
sound. At Amgen, our review made a great company
even better. I am fully confident we have an inde-
pendent, experienced, fully-engaged board that asks
probing questions, holds management accountable,
and represents stockholder interests extremely well.
Amgen’s stock performance in 2002 was good
compared to other stocks, but frustrating in the face
of what we and others recognize as outstanding
operating results. We are confident, however, that
by delivering strong and consistent results, Amgen
will be well-positioned to deliver very attractive stock-
holder gains when the current economic and stock
market environments improve.
Kevin W. Sharer
Chairman and
Chief Executive Officer
March 3, 2003
Amgen achievements
●Received approval for Neulasta™
(pegfilgrastim) in the United States and
Europe for use in the management of
chemotherapy-induced neutropenia.
●Launched Neulasta™in the United States –
considered to be the most successful
injectable product launch ever.
●Received approval for Aranesp
®
(darbepoetin
alfa) in the United States and Europe for the
treatment of chemotherapy-induced anemia.
●Successfully acquired and integrated
Immunex Corporation.
●Received approval for ENBREL
®
(etanercept)
in the United States for reducing signs and
symptoms of active arthritis in psoriatic
arthritis patients.
●Submitted multiple regulatory filings for
expansion of product labels.
●Won financial damages and reimbursement
of legal costs when arbitrator found that
Johnson & Johnson had breached our license
agreement.
●Completed phase 3 studies for ENBREL
®
in
psoriasis and ankylosing spondylitis.
●Completed phase 3 study enrollment for
KGF and Cinacalcet hydrochloride.
●Received approval for the ENBREL
®
manufacturing facility in Rhode Island.
●Received approximately $2.8 billion from
issuance of 30-year, zero-coupon senior
convertible notes.
●Delivered strong financial performance.