Medtronic 2011 Annual Report Download
Download and view the complete annual report
Please find the complete 2011 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages
listed below, or by using the keyword search tool below to find specific information within the annual report.
-

1
-
2
-

3
-

4
-

5
-

6
-

7
-

8
-

9
-

10
-

11
-

12
-

13
-

14
-

15
-

16
-

17
-

18
-

19
-

20
-

21
-

22
-

23
-

24
-

25
-

26
-

27
-

28
-

29
-

30
-

31
-

32
-

33
-

34
-

35
-

36
-

37
-

38
-

39
-

40
-

41
-

42
-

43
-

44
-

45
-

46
-

47
-

48
-

49
-

50
-

51
-

52
-

53
-

54
-

55
-

56
-

57
-

58
-

59
-

60
-

61
-

62
-

63
-

64
-

65
-

66
-

67
-

68
-

69
-

70
-

71
-

72
-

73
-

74
-

75
-

76
-

77
-

78
-

79
-

80
-

81
-

82
-

83
-

84
-

85
-

86
-

87
-

88
-

89
-

90
-

91
-

92
-

93
-

94
-

95
-

96
-

97
-

98
-

99
-

100
-

101
-

102
-

103
-

104
-

105
-

106
Table of contents
-
Page 1
-
Page 2
...
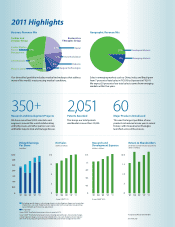
Business Revenue Mix
Cardiac and Vascular Group
Cardiac Rhythm Disease Management
Geographic Revenue Mix
Restorative Therapies Group
21% 31% 10%
Spinal
91%
Neuromodulation Diabetes Surgical Technologies
Developed Markets
9%
Emerging Markets
CardioVascular
20% 8% 3% 7%
Physio-Control
Our...
-
Page 3
... of chronic disease and changing the lives of more than 7 million patients worldwide each year. Medtronic is headquartered in Minneapolis, Minnesota; we serve patients and physicians in more than 120 countries through 45,000 employees; and we are publicly traded on the New York Stock Exchange under...
-
Page 4
... †35 Chronic Pain
Cardiovascular Diseases
6 Coronary Artery Disease 7 Heart Valve Disease 8 Aortic Disease 9 Peripheral Vascular Disease * 10 High Blood Pressure * 11 Congenital Heart Disease
Urological and Digestive Disorders
36 Overactive Bladder and Urinary Retention 37 Benign Prostatic...
-
Page 5
Dear Shareholders,
When I decided to come to Medtronic, I knew I was joining a company that has demonstrated a steadfast commitment to customers and patients throughout its history; a company with a strong Mission, culture, and business fundamentals. Given the tremendous need for what we do, I am ...
-
Page 6
... the world.
We also introduced a number of important and differentiated new therapies that are expected to help drive growth and market share in FY12. In our Cardiac and Vascular Group, we launched the first pacemaker in the United States designed for use in the MRI environment; a portfolio of ICD...
-
Page 7
... opportunity to improve human health around the world, and at the same time to create shareholder value. Given the sense of purpose and passion instilled in us by our Mission, there is no question we will contribute to combating the global burden of chronic disease. Reigniting growth by accelerating...
-
Page 8
INNOVATION HAS MANY FACES.
4
-
Page 9
... BY CHRONIC DISEASE, A GROWING PANDEMIC THAT IS STRAINING HEALTH CARE SYSTEMS AND WEAKENING GLOBAL ECONOMIES.
So we're working across borders and across disciplines to continually deliver collaborative innovations - new therapies, processes, and programs that are improving the way the world views...
-
Page 10
..., ï¬rst-line therapies - diet, exercise, and medication - aren't enough to bring their blood pressure under control. So we invested in an innovative, catheter-based treatment* for high blood pressure.
I am the face of prevention.
The treatment, called renal denervation, addresses a key contributor...
-
Page 11
Developed by our Ardian business, renal denervation is changing the way physicians help people like Monika Kraus of Homburg, Germany, better control their high blood pressure.
7
-
Page 12
Our latest spinal systems maximize the effectiveness of neurosurgeons, such as Dr. Bradford Mullin with Central Ohio Neurological Surgeons in Columbus, Ohio.
8
-
Page 13
... shoulder to prevent numbness."
Broad range of implant siïºes
We offer numerous sizes and strengths of rods to help stablize the spine so neurosurgeons can select the therapy best suited for each patient's condition and anatomy.
Powered surgical instruments
With some spinal surgeries lasting up...
-
Page 14
... driven by cardiovascular disease and diabetes epidemics in India, we're rapidly increasing our efforts in the country. We moved into a new, larger headquarters in 2011 and will be adding more than 500 employees over the next five years. We also plan to train more than 4,000 implanters and surgeons...
-
Page 15
Employees like Sarah Bandiola are helping combat chronic disease in Asia by producing much-needed cardiac devices in our new Singapore manufacturing facility.
11
-
Page 16
Dr. Robert Emery of St. Joseph's Hospital in St. Paul, Minnesota, is a leading advocate for eliminating blood transfusions during cardiac surgeries. Medtronic is helping hospitals accomplish this through an innovative education program.
12
-
Page 17
...Minnesota, which started a bloodless surgery program in early 2011. The hospital has implemented several changes in techniques and products, including the Medtronic Resting Heart System, which supports the patient's heart and lungs during surgery. The program is designed to reduce the need for blood...
-
Page 18
... better positioning of the valve. We're currently conducting clinical trials for the CoreValve System in the United States to gain FDA approval.
Transcatheter heart valves allow physicians to replace aortic valves without opening the chest. The artificial valve is delivered inside a catheter, which...
-
Page 19
At age 78, Norma Alessandria of Buenos Aires, Argentina, was considered ineligible for open-heart surgery to replace her narrowing aortic heart valve. Instead, she was able to have it replaced nonsurgically with our transcatheter aortic valve.*
15
-
Page 20
... player survived and today has a Medtronic implantable cardioverter defibrillator (ICD) to help prevent another SCA episode. The traumatic incident motivated Anderson to fast-track CPR training and he's working to get an AED at each of the school's seven athletic locations. "This issue can't be on...
-
Page 21
...acquisition-related items, non-cash charge to interest expense due to the accounting rules governing convertible debt, and certain tax adjustments(2) Dividends per share Return on equity Research and development expense Closing stock price
0.09
0.73
1.08
0.43
0.50
2.41 0.44 25.1% $ 1,239 53.60...
-
Page 22
... first quarter of fiscal year 2011, due to changes in how we internally manage and report the results of these businesses, we began to operate under two reportable segments and two operating segments, the Cardiac and Vascular Group (composed of the Cardiac Rhythm Disease Management (CRDM), CardioVas...
-
Page 23
...market growth rates, and redu ced reimbursement in certain countries including Japan, where R-Zone and foreign reference pricing changes resulted in a de cline in our selling prices. Net sales growth for fiscal year 2011 was also impacted by a CRDM competitor's stop shipment in the prior fiscal year...
-
Page 24
..., in-process research and deve lopment (IPR&D), contingent consideration, warranty obligations, product liability, self-insurance, pension and post-retirement obligations, sales returns and discounts, stockbased compensation, valuation of equity and debt securities, and income tax reserves are...
-
Page 25
... the fiscal year ended April 29, 2011 of approximately $43 million. See the discussion of our tax rate and tax adjustments in the "Income Taxes" section of this management's discussion and analysis. Valuation of IPR&D, Contingent Consideration, Goodwill, and Other Intangible Assets When we acquire...
-
Page 26
...Other CARDIAC RHY THM DISEASE MANAGEMENT Coronary and Peripheral Struc tural Heart Endovascular CARDIOVASCULAR PHYSIO-CONTROL TOTAL CARDIAC AND VASCULAR GROUP Core Spinal Biologics SPINAL NEUROMODULATION DIABETES SURGICAL TECHNOLOGIES TOTAL RESTORATIVE THERAPIES GROUP TOTAL
In fiscal years 2011 and...
-
Page 27
...-Ds. Net sales growth for fiscal year 2010 was also impac ted by a CRDM competitor's stop shipment in the fourth quarter and due to an extra selling week in the first quarter. The Secura ICDs and Consulta CRT-Ds feature OptiVol Fluid Status Monitoring (OptiVol) and Conexus wireless technology which...
-
Page 28
... 3D platform to deliver a full suite of single, dual, and triple chamber defibrillators that include SmartShock Technology, a family of new Medtronic-exclusive algorithms that reduces the delivery of inappropriate shocks, which is a leading clinical request from physicians. • Continued and future...
-
Page 29
...date we have been successful in gaining share with this stent platform in those geographies where the product has been approved. • Future growth in the U.S. from the launch of the Endurant Abdominal Stent Graft System, which was approved and launched in the third quarter of fiscal year 2011. Early...
-
Page 30
..., urinary retention, and bowel control (outside the U.S.), partially offset by declines in pain management products. Diabetes net sales for fiscal year 2011 were $1.347 billion, an increase of 9 percent over the same period in the prior fiscal year. Net sales increased worldwide led by international...
-
Page 31
... was also an increase in worldwide net sales of CGM systems worldwide. During fiscal year 2010, we reached settlement with the suppliers involved in the July 2009 recall of specific lots of Quick-set infusion sets that are used with the MiniMed Paradigm insulin pumps. T he re c all was initiated be...
-
Page 32
...the treatment of the symptoms of overactive bladder and urinary retention. InterStim T herapy for Bowe l Contro l is also approved in Europe. InterStim Therapy for Bowel Control was approved by the FDA in the fourth quarter of fiscal year 2011 and launched in the first quarter of fis c a l year 2012...
-
Page 33
... launched in the U.S. in the fourth quarter of fiscal year 2010. The launch of this system extended our line of sensor-augmented therapy options available on the market. • Given the elective nature of an insulin pump and CGM for the management of diabetes and the possible high out-of-pocket c osts...
-
Page 34
...In fis c al years 2011 and 2010, there were no special charges. In fiscal year 2009, consistent with our ongoing commitment to improving the health of people and communities throughout the world, we recorded a $100 million contribution to The Medtronic Foundation, which is a related party non-profit...
-
Page 35
...was also designed to further consolidate manufacturing
of CardioVascular products, streamline distribution of products in select businesses, and reduce general and administrative costs in our corporate functions. In the first quarter of fiscal year 2009, as a continuation of the gl obal realignment...
-
Page 36
... under their 1997 settlement and license agreement relating to coronary angioplasty stent design and balloon material patents. The settlement amount was paid in May 2009. The third charge in fiscal year 2009 of $229 million related to litigation with Cordis Corporation (Cordis), a subsidiary of...
-
Page 37
... these therapies. See the "Acquisitions" section of this management's discussion and analysis for detailed discussion of each material acquisition in fiscal years 2011, 2010, and 2009. Certain Tax Adjustments We cl assify the materia l re cognition or dere cognition of un certain tax positions as...
-
Page 38
...on trading se curities, changes in the fair value of interest rate derivative instruments,
and the net realized gain or loss on the sale or impairment of availab l e-for-sal e debt se curities. In fis c al year 2011, interest expense, net was $278 million, as compared to $246 million in fiscal year...
-
Page 39
... Irish research and development credit claims, the deductibility of a settlement expense, the finalization of certain foreign and domestic tax returns and changes to uncertain tax position reserves. This benefit was partially offset by the $15 million tax cost associated with the U.S. health care...
-
Page 40
... to take advantage of favorab l e interest rate environments or other market conditions. At April 29, 2011, our Standard and Poor's Ratings Group and Moody's Investors Service ratings remain unchanged as compared to the fiscal year ended Apri l 30, 2010, with l ong-term debt ratings of AA- and A1...
-
Page 41
... ac tivitie s Financing ac tivities Effec t of exchange rate changes on cash and cash equivalents Net change in cash and cash equivalents
Operating Activities Our net cash provided by operating activities was $3.741 billion for the fiscal year ended April 29, 2011 compared to $4.131 billion for the...
-
Page 42
... in fiscal year 2010 as compared to fiscal year 2009, primarily due to the debt issuance of $3.000 billion during fiscal year 2010. Our cash returned to shareholders
in the form of dividends and the repurchase of common stock was $335 million higher in fiscal year 2010 as compared to fiscal year...
-
Page 43
... $81 million payment of a revenue milestone to the former shareholders of CoreValve, Inc. in accordance with the fiscal year 2009 acquisition agreement. (4) Interest payments in the table above reflect the interest on our outstanding debt, including $1.000 billion of 2011 Senior Notes, $3.000...
-
Page 44
... requested adjustment to the exercise price of the warrants from $75.30 to $74.71 pursuant to the antidilution provisions of the warrants relating to our payment of dividends to shareholders of our common stock. As of April 29, 2011 and April 30, 2010, we had interest rate swap agreements designated...
-
Page 45
... pro-rata share in Ardian, plus potential future commercia l mil estone payments equa l to the annua l revenue growth beginning in fiscal year 2012 through the end of our fiscal year 2015. We recorded a gain of $85 million on our previously held investment. On November 16, 2010, we acquired Osteote...
-
Page 46
... technologies for the interventional treatment of cardiovascular disease. In April 2009, we acquired CoreValve. Under the terms of the agreement, the transac tion included an initial up-front payment of $700 million plus potential additional payments contingent upon achievement of certain clinical...
-
Page 47
... 2011. T he prior year a l so had approximately $200 million of revenue benefit from the extra week in the first quarter of fiscal year 2010. Outside the U.S., net sa l es growth was l ed by strong doub l e-digit growth in CardioVascular, Diabetes, and Surgical Technologies. CardioVascular net sales...
-
Page 48
... approval of new products; increased presence in new markets, as
we ll as c hanges in the market and our market share; the completion of planned acquisitions, divestitures and strategic investments, as well as integration of acquired companies into our operations; the resolution of tax matters; the...
-
Page 49
...l uation, management concluded that the Company's internal contro l over financial reporting was effec tive as of April 29, 2011. Our internal control over financial reporting as of April 29, 2011 has been audited by Pri c ewaterhouseCoopers LLP, an independent registered public accounting firm, who...
-
Page 50
... each of the three fiscal years in the period ended April 29, 2011 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of Apri l 29...
-
Page 51
Consolidated Statements of Earnings
Fiscal Year
(in millions, except per share data)
2011 $ 15,933 3,912 1,508 5,533 - 261 ... 2,070
Net sales Costs and expenses: Cost of products sold Research and development expense Selling, general, and administrative expense Special charges Restructuring charges...
-
Page 52
... Total assets Liabilities and Shareholders' Equity Current liabilities: Short-term borrowings Accounts payable Accrued compensation Accrued income taxes Other accrued expenses Total current liabilities Long-term debt Long-term accrued compensation and retirement benefits Long-term accrued income...
-
Page 53
... and award plans Adjustment for change in plan measurement date pursuant to the new authoritative guidance for accounting for defined benefit pension and other post-retirement plans Repurchase of common stock Tax benefit/(deficit) from exercise of stock-based awards Stock-based compensation Balance...
-
Page 54
... Issuance of long-term debt Payments on long-term debt Dividends to shareholders Issuance of common stock Excess tax benefit from exercise of stock-based awards Repurchase of common stock Net cash provided by (used in) financing activities Effect of exchange rate changes on cash and cash equivalents...
-
Page 55
.... Primary products include those for cardiac rhythm disorders, cardiovascular disease, neurological disorders, spinal conditions and musculoske letal trauma, urological and digestive disorders, diabetes, and ear, nose, and throat conditions. T he Company is headquartered in Minneapo lis, Minnesota...
-
Page 56
... net assets exceed the estimated fair value of the reporting unit. The estimated fair value is determined using a discounted future cash f low analysis. The Company completed its annual goodwill impairment test in the third quarter of fiscal years 2011, 2010, and 2009 and determined that no goodwill...
-
Page 57
... for the discount rate, retirement age, compensation rate increases, and the expec ted return on plan assets. Post-retirement medical benefit costs include assumptions for the discount rate, retirement age, expec ted return on plan assets, and health care cost trend rate assumptions.
Medtronic, Inc...
-
Page 58
... Consolidated Financial Statements
(continued)
T he Company evaluates the dis count rate, retirement age, compensation rate increases, expected return on plan assets, and health care cost trend rates of its pension benefits and postretirement benefits annually. In evaluating these assumptions, many...
-
Page 59
... tax expense/(benefit) related to the net change in retirement obligations was $3 million, $(112) million, and $(109) million in fiscal years 2011, 2010, and 2009, respectively. The Company adopted measurement date authoritative guidance for defined benefit plans in the fourth quarter of fiscal year...
-
Page 60
...sto ck-based awards granted under stock-based compensation plans and shares committed to be purchased under the employee stock purchase plan. The table below sets forth the computation of basic and diluted earnings per share:
Fiscal Year
(in millions, except per share data)
2011 $3,096
2010 $3,099...
-
Page 61
... years 2011 and 2010, there were no special charges. In fiscal year 2009, consistent with the Company's commitment to improving the health of people and communities throughout the world, the Company recorded a $100 million contribution to The Medtronic Foundation, which is a related party non-profit...
-
Page 62
... under their 1997 settlement and license agreement relating to coronary angioplasty stent design and balloon material patents. The Company paid the settlement in May 2009. The third charge in fiscal year 2009 of $229 million related to litigation with Cordis Corporation (Cordis), a subsidiary of...
-
Page 63
...within the CardioVascular business. In connection with the fiscal year 2011 initiative, as of the end of the fourth quarter of fis c a l year 2011, the Company had identified approximately 2,100 positions for elimination to be achieved through voluntary early retirement packages offered to employees...
-
Page 64
... was also designed to further consolidate manufacturing of CardioVascular products, streamline distribution of products in select businesses and to reduce general and administrative costs in the Company's corporate functions. In the first quarter of fiscal year 2009, as a continuation of the global...
-
Page 65
...pro-rata share in Ardian, plus potential
future commercia l mil estone payments equa l to the annua l revenue growth beginning in fiscal year 2012 through the end of the Company's fiscal year 2015. Based upon the acquisition valuation, the Company acquired $55 million of technology-based intangible...
-
Page 66
..., manufacturer, and marketer of products and services focused on cardiac surgery, including heart valves and surgical cryoablation te chno logy. Under the terms of the agreement, AT S Medic al shareholders received $4.00 per share in cash for each share of ATS Medical common stock that they owned...
-
Page 67
... costs, of $700 million plus potential additional payments contingent upon achievement of certain clinical and revenue milestones. CoreValve develops percutaneous, catheter-based transfemoral aortic valve replacement produc ts that are approved in certain markets outside the U.S.
Medtronic...
-
Page 68
.... The IPR&D was expensed on the date of a cquisition and primaril y re l ates to the future l aun ch of CoreValve's catheter-based transfemoral aortic valve into the U.S. market. For purposes of valuing the acquired IPR&D, the Company estimated total costs to complete of approximately $80 million at...
-
Page 69
... catheter designed spe cifically to treat atrial fibrillation and is currently approved in certain markets outside the U.S. In addition, the Arctic Front system was approved in the U.S. in the third quarter of fiscal year 2011. In connec tion with the acquisition of CryoCath, the Company acquired...
-
Page 70
...a l year 2011, the Company decreased the undiscounted future contingent consideration by $81 million to ref lect the achievement and subsequent payment of a revenue milestone to the former shareholders of CoreValve in accordance with the fiscal year 2009 acquisition agreement. At April 29, 2011, the...
-
Page 71
... (38)
(a) Includes available-for-sale debt securities. (b) Includes marketable equity securities, cost method, equity method, exchange-traded funds, and other investments. (c) As a result of the Ardian acquisition that occurred during fiscal year 2011, the Company recognized an $85 million non-cash...
-
Page 72
..., the Company transferred investments accounted for as cost method investments with a cost basis of $163 million to available-for-sale marketab l e equity se curities, due to restric tions on a pub lic company investment being within one year from expiration as well as the initial public offering of...
-
Page 73
... securities and debt se c urities that are cl assified and a ccounted for as trading, available-for-sale, and derivative instruments. Derivatives include cash f low hedges, freestanding derivative forward contracts, and interest rate swaps. These items are marked-to-market at each reporting period...
-
Page 74
... include highly liquid government bonds within the U.S. government and agenc y securities, marketable equity securities, and exchange-traded funds for which quoted market prices are available. In addition, the Company has determined that foreign currenc y forward contrac ts are included in Level...
-
Page 75
...of debt discounts and derivative/hedging activity. 7. Good w i ll and Other Intan g i bl e Assets The changes in the carrying amount of goodwill for fiscal years 2011 and 2010 are as follows:
Cardiac and Vascular Group $ 1,392 155 (5) 46 $ 1,588 1,046 25 20 $2,679 Restorative Therapies Group $ 6,803...
-
Page 76
...:
(in millions)
Fiscal Year 2012 2013 2014 2015 2016 Thereafter
Amortization Expense $ 321 304 294 279 268 973 $2,439
8. Financin g Arran g ements Debt consisted of the following:
April 29, 2011
(in millions, except interest rates)
April 30, 2010 Effective Interest Rate Payable $ - - 65 2,200...
-
Page 77
...6.50% 5.55% - - 4.21% 5.60% - Effec tive Interest Rate - 6.03% 4.50% 3.00% 4.76% - 5.61% 4.47% - 6.52% 5.56% - - - - -
Maturity by Fiscal Year 2012-2022 2013 2014 2015 2016 2016 2019 2020 2021 2039 2040 2013-2021 2011-2016 2013-2025 2013 2011-2013
Payable $ 15 2,200 550 1,250 600 500 400 1,250 500...
-
Page 78
...its own stock and classified in shareholders' equity in its statement of financial position. The Company concluded that the purchased call option contrac ts and the warrant contrac ts should be accounted for in shareholders' equity. Effe c tive the first day of the Company's fis c a l year 2010, the...
-
Page 79
..., and excluding the debt discount, the fair value impac t of outstanding interest rate swap agreements, and the remaining gains from terminated interest rate swap agreements are as follows:
(in millions)
Fiscal Year 2012 2013 2014 2015 2016 Thereafter Total long-term debt Less: Current portion...
-
Page 80
...g ement T he Company uses operational and economic hedges, as well as c urren c y ex c hange rate derivative c ontra c ts and interest rate derivative instruments to manage the impac t of currenc y exchange and interest rate changes on earnings and cash f lows. In order to minimize earnings and cash...
-
Page 81
... agreements with a conso l idated notiona l amount of $750 mi ll ion, whi c h were designated as fair value hedges of fixed interest rate obligations under the Company's 2011 Senior Notes due 2016 and 2021. The Company pays variable interest equal to the London Interbank Offered Rate (LIBOR) plus...
-
Page 82
...million interest rate swap agreement, the Company pays variable interest equal to the three-month LIBOR plus 198.10 basis points and it receives a fixed interest rate of 4.500 percent.
In June 2009, the Company entered into two five-year fixed-tof loating interest rate swap agreements with notional...
-
Page 83
... includes the expense asso ciated with the interest that the Company pays on its outstanding borrowings, including short- and long-term instruments, changes in the fair value of interest rate derivative instruments, and the amortization of debt issuance costs and debt discounts.
Medtronic, Inc. 79
-
Page 84
... from time to time to support the Company's stock-based compensation programs and to return capital to shareholders. The Company repurchased approximately 30.1 million and 27.0 million shares at an average price of $37.86 and $38.10, respectively, during fiscal years 2011 and 2010. As of April...
-
Page 85
... most recent quarterly dividend rate, by the closing stock price on the grant date.
Stock-Based Compensation Expense Upon the adoption of the fair value re cognition provisions of U.S. GAAP for accounting for stock-based compensation, the Company changed its method of recognition and now recognizes...
-
Page 86
... issues new shares when stock option
awards are exercised. Cash received from the exercise of stock options for the fiscal year ended April 29, 2011 was $21 million. The Company's tax benefit related to the exercise of stock options for fiscal year 2011 was $1 million. Unrecognized compensation...
-
Page 87
...tax accounting, known as "temporary differences." T he Company records the tax effect of these temporary differences as "deferred tax assets" and "deferred tax l iabi l ities." Deferred tax assets generally represent items that can be used as a tax deduction or credit in a tax return in future years...
-
Page 88
...Years
(in millions)
Deferred tax assets: Inventory (intercompany profit in inventory and excess of tax over book valuation) Stock-based compensation Accrued liabilities Net operating loss and credit carryforwards Other Federal and state benefit on uncertain tax positions Pension and post-retirement...
-
Page 89
... if changes to the Company's allocation are required between jurisdic tions with different tax rates. Tax authorities periodically review the Company's tax returns and propose adjustments to the Company's tax filings. The IRS has settled its audits with the Company for all years through fiscal year...
-
Page 90
... plan. A status of the Company's benefit plans was $253 million and $411 million, respectively.
The change in benefit obligation and funded status of the Company's employee retirement plans are as follows:
U.S. Pension Benefits Fiscal Year
(in millions)
Non-U.S. Pension Benefits Fiscal Year 2011...
-
Page 91
... amortized from accumulated other comprehensive (loss)/income into net periodic benefit cost, before tax, in fiscal year 2012 are as follows:
U.S. Pension Benefits $ (1) 45 $44 Non-U.S. Pension Benefits $2 4 $6 PostRetirement Benefits $- 4 $ 4
(in millions)
(in millions)
Net ac tuarial loss/(gain...
-
Page 92
... - net periodic benefit cost: Discount rate Expec ted return on plan assets Rate of compensation increase Initial health care cost trend rate Initial health care cost trend rate 2010 2009 2011 Non-U.S. Pension Benefits Fiscal Year 2010 2009 2011 Post-Retirement Benefits Fiscal Year 2010 2009
pre...
-
Page 93
... Leve l 2 se c urities. Consequent l y, the Company transferred registered investment companies from Level 1 to Level 2. There were no significant transfers from Level 1 or 2 to Level 3 during the fiscal years ended April 29, 2011 or April 30, 2010.
Retirement Benefit Plan Asset Fair Values T he fo...
-
Page 94
...government securities Corporate debt securities Medtronic, Inc. common stock Other common stock Fixed income mutual funds Partnership units Total Other items to reconcile to fair value of plan assets
(in millions)
Registered investment companies Insurance contrac ts Partnership units
(6) $158
90...
-
Page 95
... plan and approximately $28 million to fund postretirement benefits. Internationally, the Company contributed approximately $102 million for pension benefits during fiscal year 2011. During fiscal year 2012, the Company anticipates that its contribution for pension benefits and post-retirement...
-
Page 96
... on the amounts reported for the health care plans. A one-percentagepoint change in assumed health care cost trend rates would have the following effects:
(in millions)
15. L eases The Company leases office, manufacturing, and research facilities and warehouses, as well as transportation, data...
-
Page 97
... District Court for the District of New Jersey, alleging that Medtronic's Endeavor drug-eluting stent infringes three U.S. "Morris" patents alleged to be owned by Wyeth and exclusively licensed to Cordis. A trial date has been set for January 9, 2012. T he Company is indemnified for the claims made...
-
Page 98
... parties subsequently reached an adjusted settlement agreement pursuant to which Medtronic waived its
right to cancel the agreement and agreed to pay a total of $221 million to resolve over 14,000 filed and unfiled claims. In the se cond quarter of fiscal year 2011, the Company re corded an expense...
-
Page 99
... Mirowski in May 2011. Other Matters On October 14, 2010, the Company received a subpoena issued by the United States Attorney's Office for the Western District of New York pursuant to the Health Insurance Portability & Accountability Ac t of 1996 (HIPAA), relating to the Company's sales, marketing...
-
Page 100
.... On September 5, 2008, Medtronic re ceived a subpoena from the Office of Inspe c tor General for the Department of Health and Human Services in the Distric t of Minnesota, requesting produc tion of substantially the same materials covered in the 2005 Massachusetts subpoena. T he Company is fully...
-
Page 101
... Therapies Group Total Net Assets of Reportable Segments Short-term borrowings Long-term debt Corporate Total Net Assets
Geographic Information Net sales to external customers by geography are as follows:
(in millions)
United Asia Other ConsoliStates Europe Pacific Foreign dated
Fiscal Year 2011...
-
Page 102
... Financial Data
Fiscal Year
(in millions, except per share data)
2011
2010
2009
2008
2007
Operating Results for the Fiscal Year: Net sales Cost of products sold Gross margin percentage Research and development expense Selling, general, and administrative expense Special charges Restructuring...
-
Page 103
... year 2011 and 20.50 cents per share for fiscal year 2010. Stock Transfer Agent and Registrar Wells Fargo Shareowner ServicesSM ac ts as transfer agent and registrar, dividend paying agent, and direc t stock purchase plan agent for Medtronic and maintains all shareholder records for the Company...
-
Page 104
..., Chief Ethics and Compliance Officer
Robert C. Pozen
Chairman, MFS Investment Management Director since 2004
Caroline Stockdale
Senior Vice President, Chief Human Resource Officer
Jean-Pierre Rosso
Chairman, World Economic Forum USA Director since 1998
Catherine M. Szyman
Medtronic Corporate...
-
Page 105
Our Mission
MISSION
• To contribute to human welfare by application of biomedical engineering in the research, design, manufacture, and sale of instruments or appliances that alleviate pain, restore health, and extend life.
MISSION
• Contribuer au bien-être de l'homme en appliquant les ...
-
Page 106
.... 710 Medtronic Parkway Minneapolis, MN 55432-5604 USA Tel: 763.514.4000 Fax: 763.514.4879
International Headquarters Medtronic International, Ltd. 49 Changi South Avenue 2 Nasaco Tech Centre Singapore 486056 Singapore Tel: 65.6436.5000 Fax: 65.6776.6335
The Medtronic 2011 Annual Report is printed...















