Amgen 2001 Annual Report Download
Download and view the complete annual report
Please find the complete 2001 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
A Life Worth Living
Amgen
2001 Annual Report
Table of contents
-
Page 1
Amgen 2001 Annual Report A Life Worth Living -
Page 2
... Products and Product Candidates Management's Discussion and Analysis of Financial Condition and Results of Operations Quantitative and Qualitative Disclosures About Market Risk Consolidated Financial Statements Report of Ernst & Young LLP, Independent Auditors Board of Directors and Executive... -
Page 3
... two signiï¬cant new products. We made promising advances in clinical and preclinical research. We brought new leadership into key areas of our business. We announced an acquisition with great promise. And we affirmed our core values and began to transform key operating processes to prepare for... -
Page 4
... fourth quarter of 1999, the Company offered extended payment terms on limited shipments of EPOGEN® (Epoetin alfa) and NEUPOGEN® (Filgrastim) to certain wholesalers. These Year 2000 related sales totaled $45 million, or $0.02 per share, in 1999. The amount in 2001 is primarily related to the costs... -
Page 5
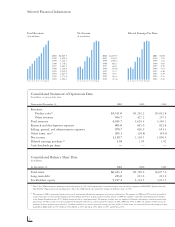
Research and Development Expenses ($ in millions) Stockholders' Equity ($ in millions) Amgen Staff 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 92 93 94 95 96 97 98 99 00 01 $865.0 845.0 822.8 663.3 630.8 528.3 451.7 323.6 255.3 182.3 92 93 94 95 96 97 98 99 00 01 2001...00 01 2001 2000 1999... -
Page 6
...to key areas of our business as we scale up Amgen to meet the challenges of a growing product line and an increasingly ambitious research and development program. We've added new senior management to the company's leadership ranks in a number of areas. Roger Perlmutter joined Amgen as executive vice... -
Page 7
... such as rheumatoid arthritis. It is a blockbuster therapeutic that has the potential to generate product sales of more than $3 billion annually by 2005. Together, ENBREL®, EPOGEN® (Epoetin alfa), NEUPOGEN®, Aranesp™, Kineret™, and Neulasta™ represent a product line unparalleled in... -
Page 8
... Worth Making up Amgen to meet the demands of our tremendous "Scaling growth opportunities is a m u l t i - f a c e t e d c h a l l e n g e . It touches every part of our business and every process we have. The capital markets are watching. They want to see Amgen succeed in delivering therapeutics... -
Page 9
... and development programs, the lifeblood of a successful human therapeutics enterprise. Research and development spending in 2001 was 25% of total product sales, among the highest levels in the biotechnology and pharmaceuticals industries. This is the seventh consecutive year Amgen's research and... -
Page 10
... the employee stock option and the stock purchase plans. Our stock repurchase program also represents one measure of our conï¬dence in the long-term value of Amgen's stock. In 2001, Amgen repurchased 12.7 million shares of its common stock at an average price of $58 per share, at a total cost of... -
Page 11
...people are believed to have low red blood cell production, which results in anemia. But these estimates are just a starting point. The debilitating impact of anemia, which causes fatigue, impaired cognitive and physical functioning, and may contribute to cardiovascular disease, occurs in a number of... -
Page 12
... Australia, and New Zealand for the treatment of anemia associated with chronic renal failure, Amgen's ï¬rst area of focus for the new therapeutic. For chronic renal failure patients, both on dialysis and not on dialysis, the new molecule retains the efficacy of EPOGEN® (Epoetin alfa) while adding... -
Page 13
...Before the development of EPOGEN®, many of these patients suffered from chronic anemia and could not maintain vitality without regular blood transfusions. Today, more than 200,000 dialysis patients in the United States receive EPOGEN® therapy. Since launching EPOGEN®, Amgen has worked diligently... -
Page 14
...sure. But we must also engage the market on its own terms, addressing cost and relative efficacy in addition to long-term patient outcomes. George Morrow Executive Vice President, Worldwide Sales and Marketing Markets Worth Developing does brand-building have to do with improving "What patients... -
Page 15
... year. In the United States alone, the number exceeds one million. Although treatments are growing in effectiveness and improving the survival rates for many types of cancer, the overall growth rate for new cancer diagnoses worldwide is estimated at more than 2% a year, outpacing the 1.7% estimated... -
Page 16
... of chemotherapy and NEUPOGEN®, as well as feedback on patient outcomes. This information has been particularly useful in helping physicians optimize treatment alternatives in the most costefficient manner. Neulasta ™, Amgen's new white blood cell booster, received U.S. regulatory approval early... -
Page 17
... tumor cells. With substantial capabilities in a variety of scientiï¬c approaches, Amgen researchers are studying small molecules and human antibodies in addition to human proteins and growth factors as potential new therapeutics. In 2000, Amgen licensed a novel cancer therapeutic antibody... -
Page 18
...new ground. Ten years ago, Amgen helped pioneer commercial production of recombinant human proteins. We built the ï¬rst multi-product human protein manufacturing facility. Today, we're laying the foundation to signiï¬cantly scale up production. Manufacturing biologically derived human therapeutics... -
Page 19
Inflammation More than 6 million people worldwide live with rheumatoid arthritis. It is one of several debilitating ... invading organisms and help repair damaged tissues. In diseases such as rheumatoid arthritis, control mechanisms fail and inï¬,ammatory reactions are directed against normal,... -
Page 20
...has been a target of Amgen's research programs for more than a decade. The company has launched its ï¬rst therapeutic speciï¬cally targeted at inï¬,ammation and the disease most commonly associated with its destructive effects, rheumatoid arthritis. In November 2001, Kineretâ„¢ was approved in the... -
Page 21
... Amgen's potential acquisition of Immunex will bring with it a roster of experienced research talent in inflammation. Amgen added more than 350 new staff members across a range of disciplines to its overall talent base during 2001. In late 2001, Amgen announced plans to acquire Immunex Corporation... -
Page 22
...the heart of that process is good decision-making. Executive Vice President, Research and Development Roger Perlmutter, MD, PhD Science Worth Pursuing is the l i f e b l o o d of any therapeutics endeavor, but "R&D it's much more than that at Amgen. It goes to the very core of our identity. " To... -
Page 23
..., Amgen continues to play a leadership role in the search for breakthrough human therapeutics. The company pursues research organized around four therapeutic areas -nephrology, oncology, inï¬,ammation, and neurology/metabolism. These internal programs are enhanced and expanded through external... -
Page 24
... structures within the cell could yield therapeutic alternatives to larger, naturally occurring proteins. Amgen uses new techniques in robotics and miniaturization to synthesize and test thousands of these small molecules quickly and cost-efficiently. Amgen's genomics research program led to the... -
Page 25
... of product candidates for Amgen that can complement internal research and development activities. In recent months, the company has entered into several new agreements with external groups to extend and enhance the value of its internal research programs. In December 2001, Amgen agreed to work with... -
Page 26
Amgen Products and Product Candidates Therapeutic Areas Products/Product Candidates Phase 1 Phase 2 Development Phase Phase 3 Filed Approved Nephrology Anemia Anemia Secondary hyperparathyroidism EPOGEN® (Epoetin alfa) Aranesp™ (darbepoetin alfa) Calcimimetics Program Hematology & ... -
Page 27
...of stock by Amgen pursuant to the employee stock purchase plan provided $277.7 million and $333.7 million of cash in 2001 and 2000, respectively. Proceeds from the exercise of employee stock options will vary from period to period based upon, among other factors, ï¬,uctuations in the market value of... -
Page 28
... program and the proposed acquisition of Immunex Corporation ("Immunex") (see "Proposed Merger with Immunex"). However, the Company may raise additional capital from time to time. Total Assets Results of Operations Product sales Product sales primarily consist of sales of EPOGEN® (Epoetin... -
Page 29
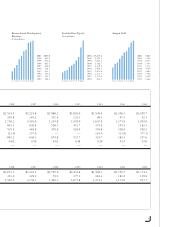
... Kirin-Amgen, Inc. In 2000, corporate partner revenues were $246.2 million, an increase of $84.8 million Total Product Sales or 53% over the prior year. ($ in millions) This increase was primarily due to amounts earned from Kirin-Amgen, Inc. related to the development program for Aranespâ„¢. Royalty... -
Page 30
... spillover arbitration with Johnson & Johnson, 2) a write-off of acquired in-process research and development associated with the acquisition of Kinetix Pharmaceuticals, Inc., and 3) a charitable contribution to the Amgen Foundation. In 1999, other items, net consisted of a reduction in liabilities... -
Page 31
...on Form 10-K for the year ended December 31, 2001, and in Amgen's other ï¬lings with the Securities and Exchange Commission, which sections are incorporated herein by reference. Summary of Critical Accounting Policies EPOGEN® revenue recognition The Company has the exclusive right to sell Epoetin... -
Page 32
... different amounts for recognized EPOGEN® sales. However, such differences to date have not been material. Inventory capitalization The Company capitalizes inventory costs associated with certain product candidates prior to regulatory approval, based on management's judgment of probable future... -
Page 33
...Interest Rate Sensitivity Principal Amount by Expected Maturity as of December 31, 2001 Dollars in millions Average Interest Rate 2002 2003 2004 2005 2006 Thereafter Total Fair Value 12/31/01 Available-for-sale debt securities Interest rate Commercial paper obligations Interest rate Long-term debt... -
Page 34
...) Years ended December 31, 2001 2000 1999 Revenues: Product sales Corporate partner revenues Royalty income Total revenues Operating expenses: Cost of sales Research and development Selling, general and administrative Loss of afï¬liates, net Other items, net Total operating expenses Operating... -
Page 35
... Current assets: Cash and cash equivalents Marketable securities Trade receivables, net of allowance for doubtful accounts of $21.4 in 2001 and $21.2 in 2000 Inventories Other current assets Total current assets Property, plant, and equipment at cost, net Other assets $ 689.1 1,973.1 497.2 355... -
Page 36
... purchase plan Tax beneï¬ts related to employee stock options Issuance of common stock for the acquisition of Kinetix Pharmaceuticals, Inc. Repurchases of common stock Balance at December 31, 2000 Comprehensive Income: Net income Other comprehensive loss, net of tax: Unrealized losses on securities... -
Page 37
... from sales of marketable securities Purchases of marketable securities Other Net cash used in investing activities Cash ï¬,ows from ï¬nancing activities: Net proceeds from issuance of common stock upon the exercise of employee stock options and in connection with an employee stock purchase plan... -
Page 38
...Notes to Consolidated Financial Statements December 31, 2001 Note 1: Summary of signiï¬cant accounting policies Business Amgen Inc. ("Amgen" or the "Company") is a global biotechnology company that discovers, develops, manufactures, and markets human therapeutics based on advances in cellular and... -
Page 39
... sales Product sales primarily consist of sales of EPOGEN® (Epoetin alfa), Aranesp™ (darbepoetin alfa), and NEUPOGEN® (Filgrastim) (see Note 10, "Segment information"). The Company has the exclusive right to sell Epoetin alfa for dialysis, certain diagnostics and all non-human, non-research... -
Page 40
... clinical manufacturing costs, contract services and other outside costs, and costs to acquire in-process research and development projects and technologies which have no alternative future use (see Note 11, "Kinetix acquisition"). Research and development expenses also include such costs related to... -
Page 41
... stock purchase plans The Company's employee stock option and stock purchase plans are accounted for under Accounting Principles Board Opinion ("APB") No. 25, "Accounting for Stock Issued to Employees" (see Note 7, "Employee stock option, stock purchase, and deï¬ned contribution plans"). Earnings... -
Page 42
... 2000 1999 Termination of collaboration agreements $203.1 Legal award, net - Write-off of acquired in-process research and development (see Note 11, "Kinetix acquisition") - Amgen Foundation contribution - $203.1 $ - (73.9) $ - (49.0) 30.1 25.0 $(18.8) - - $(49.0) Termination of collaboration... -
Page 43
..., the Company granted Johnson & Johnson's afï¬liate, Ortho Pharmaceutical Corporation, a license relating to certain patented technology and knowhow of the Company to sell a genetically engineered form of recombinant human erythropoietin, called Epoetin alfa, throughout the United States for all... -
Page 44
... Expenses capitalized for tax purposes Acquired net operating loss and credit carryforwards Credit carryforwards Fixed assets Other Total deferred tax assets Valuation allowance Net deferred tax assets Deferred tax liabilities: Purchase of technology rights Marketable securities and investments... -
Page 45
...at any time prior to the public announcement that a 10% position has been acquired. Stock repurchase program The Company has a stock repurchase program primarily to reduce the dilutive effect of its employee stock option and stock purchase plans. Stock repurchased under the program is intended to be... -
Page 46
... options granted after 1994. Pro forma information regarding net income and earnings per share shown below was determined as if the Company had accounted for its employee stock options and shares sold under its employee stock purchase plan under the fair value method of that statement. The fair... -
Page 47
... has an employee stock purchase plan whereby, in accordance with Section 423 of the Internal Revenue Code, eligible employees may authorize payroll deductions of up to 10% of their salary to purchase shares of the Company's common stock at the lower of 85% of the fair market value of common stock on... -
Page 48
... values. At December 31, 2001 Geographic information Outside the U.S., the Company sells NEUPOGEN® in the European Union ("EU"), Canada, and Australia. Outside the U.S., the Company sells Aranesp™ in most countries in the EU, Australia, and New Zealand. Information regarding revenues and long... -
Page 49
... expected to generate future molecules that may be developed into human therapeutics, as well as in-process research projects. The analysis resulted in valuing the acquired base technology at $36.6 million, which was capitalized and will be amortized on a straight-line basis over a 15 year period... -
Page 50
....1 million to write-off acquired in-process research and development related to the acquisition of Kinetix (see Note 11, "Kinetix acquisition"). In addition, the Company made a contribution of $25 million to the Amgen Foundation (see Note 4, "Other items, net - Amgen Foundation contribution"). After... -
Page 51
...E N 2 0 0 1 A N N UA L R E PORT Report of Ernst & Young LLP, Independent Auditors The Board of Directors and Stockholders of Amgen Inc. We have audited the accompanying consolidated balance sheets of Amgen Inc. as of December 31, 2001 and 2000, and the related consolidated statements of operations... -
Page 52
.... Brian McNamee Senior Vice President Human Resources Franklin P. Johnson, Jr. General Partner Asset Management Partners Kevin W. Sharer Chairman of the Board Chief Executive Ofï¬cer, and President Stockholder Information Corporate Ofï¬ce One Amgen Center Drive Thousand Oaks, California 91320... -
Page 53
...R E P O RT Dora Menchaca JUNE 6, 1956 SEPTEMBER 11, 2001 Joined Amgen in 1991 Associate Director, Clinical Research PhD UCLA-Epidemiology As a scientist, friend, wife, and mother, Dora cared deeply about people. She was passionate about her work, dedicated to her family, friends, and patients, and... -
Page 54
Amgen Inc. One Amgen Center Drive Thousand Oaks, CA 91320-1799 www.amgen.com © 2002 Amgen Inc. All rights reserved. MC17249 830M/3-02 P50300-3