Medtronic 2010 Annual Report Download - page 10
Download and view the complete annual report
Please find page 10 of the 2010 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
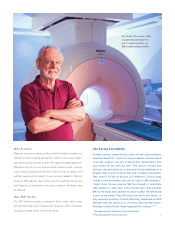
Making Devices
for Use with MRI
Medtronic introduced the world’s first
pacing system that’s designed for use
in magnetic resonance imaging (MRI)
systems—so pacemaker patients can
have access to the potentially life-saving
diagnostic tool.
Every 5 minutes, a patient with an implanted cardiac device
is denied MRI,(1) which can interfere with device operation
and damage system components.
“Doctors often must either forego the potentially life-saving
benefits of MRI or accept the significant risks associated
with scanning patients who have certain implantable
devices,” said Pat Mackin, president of our Cardiac Rhythm
Disease Management business. “According to physicians,
MRI safety and accessibility for the millions of people
who have pacemakers and other implantable devices is
a serious unmet medical need.”
Not for long.
Medtronic introduced the world’s first pacing system for use
in MRI systems. The first generation of these devices
became available in Europe in 2008, and we’re now in the
process of seeking FDA approval for a similar device in the
United States.
Medtronic designed an entire system—pulse generator,
leads, and software—that addresses the impact of the MRI
environment. One of the compatibility issues with current
systems is the possibility of pacing interference. “Electrical
stimulation devices can misinterpret MRI-generated electri-
cal noise and withhold or deliver unnecessary pacing ther-
apy,” said Jeff Burrows, MRI team leader. “We developed a
special programming feature so the device can be set to an
appropriate mode for the MRI environment.”
Another issue with current devices has to do with their leads,
which are insulated wires that run from the stimulation
device to the target area of the body—in this case,
the heart.
“The lead wires act as an antenna. They pick up energy from
the MRI, and that extra energy is carried along the lead wire,”
Burrows said. At one end, the dissipation of this extra energy
can overheat and damage human tissue. At the other end,
it can damage the device’s sensitive electronics.
Our MRI pacemaker system was designed to manage the
safe dissipation of the extra energy without compromising
therapeutic stimulation. “Our goal is to make devices that
can be used safely in an MRI environment,” Burrows said,
“while maintaining their current therapeutic value, reliability,
and safety.”
(1) Medtronic data on file.
Advisa DR MRI SureScan Pacing System*
*Not approved for marketing in the United States.
6