Eli Lilly 2010 Annual Report Download - page 4
Download and view the complete annual report
Please find page 4 of the 2010 Eli Lilly annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
2
To Our Shareholders
This year marks the 135th anniversary of the founding
of Eli Lilly and Company. It also formally marks the
beginning of a period of major patent expirations we call
“Years YZ,” when Zyprexa® goes off patent in late 2011
in the United States. For all intents and purposes, we’re
already managing through the impact of YZ in many of
our markets—including the United States, where generic
versions of our anti-cancer medicine Gemzar® entered the
market last November.
The loss of Zyprexa and other patented products will
hurt our top-line and bottom-line performance. Yet, we’ve
seen this coming and we’re prepared, with a strategy we
believe will create the greatest value for our shareholders,
employees, and society: accelerating the flow of innova-
tive medicines that provide improved outcomes for
individual patients.
We have a robust and exciting pipeline, and we advanced
a number of promising molecules in 2010.
We see strong growth ahead for many
of our current products, and we’re
among the fastest-growing compa-
nies in three important areas that
will provide countercyclical growth
opportunities through the YZ period:
Japan, key emerging markets, and our
Elanco animal health business.
Our financial strength and strong
current performance enable us to
continue to pursue business develop-
ment, to make capital investments,
and to maintain the dividend to our
shareholders. In short, Lilly is well
positioned to bridge YZ and emerge
even stronger.
In this letter, I want to provide a broad
look at how we’re approaching this
challenge with a focus on sustained growth:
•
First, I’ll review how we’re preparing for YZ in the near
term, by continuing to generate strong performance
and taking full advantage of key growth engines.
• Second, I’ll discuss how we’re using business develop-
ment to bolster revenue and cash flow in the medium
term—and, in particular, the investment we’re mak-
ing to reclaim leadership in diabetes.
• And lastly, I’ll highlight important molecules in our
pipeline—the key to our long-term future—and
discuss how we continue to reenergize our innova-
tion engine to resume growth.
Near Term: Operating effectively and accelerating our
growth engines
In 2010, Eli Lilly and Company posted strong financial
performance, highlighted by volume-driven revenue
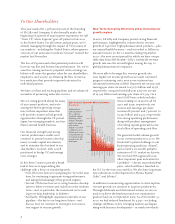
growth of 6 percent. Eight pharmaceutical products—plus
our animal health business—each exceeded $1 billion in
annual revenues. In the 12 months ending September
2010—the most recent period for which we have compa-
rable data from IMS Health—Lilly’s worldwide revenue
growth rate was the second-highest among the top 10
global pharmaceutical companies.
We were able to leverage this revenue growth into
even higher net income growth as we made continued
progress containing costs, even as we sustained our
substantial investment in R&D. Reported net income and
earnings per share increased to $5.070 billion and $4.58,
respectively, compared with full-year 2009 net income
of $4.329 billion and earnings per share of $3.94. On a
non-GAAP basis, which excludes
items totaling $0.16 and $0.48 for
2010 and 2009, respectively, net
income and earnings per share
increased 8 percent and 7 percent, to
$5.241 billion and $4.74, respectively.
Our strong operating performance,
along with prudent management
of working capital, generated some
$6.9 billion of operating cash flow.
We generated solid volume growth
in our current products in 2010. We
fended off a patent challenge to our
fastest-growing medicine, Alimta®,
and secured a six-month pediatric
extension of U.S. market exclusivity.
We received FDA approval for an-
other important pain indication for
Cymbalta®—chronic musculoskeletal
pain—which will have launched in
the U.S. by the time you read this. We also have important
new indications in development for Alimta, Byetta®,
Cialis®, and Erbitux®.
In addition to maximizing opportunities to drive top-line
revenue growth, we continue to improve productivity.
Through deliberate and determined actions, we are on
track to achieve the headcount and cost-containment
goals we laid out in September 2009. As of December 31,
2010, we had reduced headcount by 3,450—excluding
strategic additions in key emerging markets and Japan
along with business development—or nearly two-thirds
0
$1,000
$2,000
$3,000
$4,000
$5,000
Zyprexa
Cymbalta
Alimta
Humalog
Cialis
Total Animal Health
Gemzar
Humulin
Evista
Eight Products Exceed $1 Billion in Revenue
($ millions)
$2,054.2
$2,208.6
$5,026.4
$3,459.2
$1,699.4
$1,391.4
$1,024.4
$1,088.9
$1,149.4
Eight products
and one product
line—Zyprexa,
Cymbalta, Alimta,
Humalog, Cialis,
Gemzar, Humulin,
and Evista, along
with animal
health—exceeded
$1 billion in 2010.
Alimta grew 29 per-
cent primarily due
to continued sales
growth in the U.S.
and Japan.