Amgen 2007 Annual Report Download
Download and view the complete annual report
Please find the complete 2007 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Amgen 2007 Annual Report
and Financial Summary
Table of contents
-
Page 1
Amgen 2007 Annual Report and Financial Summary -
Page 2
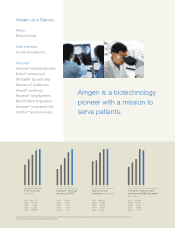
... Core business: Human therapeutics Products: Aranesp® (darbepoetin alfa) Enbrel® (etanercept) EPOGEN® (Epoetin alfa) Kepivance® (palifermin) Kineret® (anakinra) Neulasta® (pegï¬lgrastim) NEUPOGEN® (Filgrastim) Sensipar ® (cinacalcet HCl) Vectibixâ„¢ (panitumumab) Amgen is a biotechnology... -
Page 3
... acquisition (Immunex) and two other major acquisitions (Tularik and Abgenix); and the organization grew to more than 20,000 staff operating in 35 countries. Then in the ï¬rst quarter of 2007 a clinical trial, exploring the use of our anemia medicine Aranesp® in cancer patients with anemia related... -
Page 4
... function at Amgen. After starting as a research scientist, he helped to create Amgen's Process Development and Manufacturing organizations. At various times he led Sales and Marketing, Research, Information Systems, Human Resources, and most recently Operations. Dennis helped build the world's most... -
Page 5
...are ready. KEVIN W. SHARER Chairman and Chief Executive Ofï¬cer February 7, 2008 Gave more than $225 million through the Amgen Foundation, Amgen's corporate giving and product donations. Key philanthropic programs include Amgen Scholars, a scientific research program for university undergraduates... -
Page 6
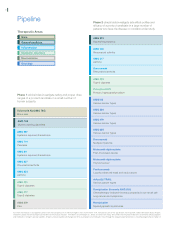
4 Pipeline Therapeutic Areas Bone General medicine Inï¬,ammation Metabolic disorders Neuroscience Oncology Phase 2 clinical trials investigate side effect proï¬les and efï¬cacy of a product candidate in a large number of patients who have the disease or condition under study. AMG 223 ... -
Page 7
...uses to patients in countries that have granted regulatory clearance. Amgen continues to develop many of its approved therapies for potential new indications. Aranesp® (darbepoetin alfa) Anemia of chronic kidney failure EPOGEN® (Epoetin alfa) Anemia of end-stage renal disease Enbrel® (etanercept... -
Page 8
...-cash amortization of acquired product technology rights, primarily ENBREL, related to the Immunex acquisition. (d ) To exclude the impact of stock option expense recorded in accordance with Statement of Financial Accounting Standards ("SFAS") No. 123R. Effective January 1, 2006, Amgen adopted SFAS... -
Page 9
..., General Counsel and Secretary Kevin W. Sharer Chairman of the Board, CEO and President Geoffrey F. Slaff Senior Vice President, Quality Stockholder Information Amgen Inc. Corporate Of ï¬ce One Amgen Center Drive Thousand Oaks, California 91320-1799 (805) 447-1000 Amgen 2007 Annual Report Summary... -
Page 10
Form 10-K 2007 Annual Report For the ï¬scal year ended December 31, 2007 -
Page 11
... One Amgen Center Drive, Thousand Oaks, California (Address of principal executive offices) (805) 447-1000 (Registrant's telephone number, including area code) Securities registered pursuant to Section 12(g) of the Act: Common stock, $0.0001 par value; preferred share purchase rights (Title of class... -
Page 12
... ...Competition ...Manufacturing and Raw Materials ...Joint Ventures and Business Relationships ...Government Regulation ...Patents and Trademarks ...Human Resources ...Executive Officers of the Registrant ...Geographic Area Financial Information ...Investor Information ...Risk factors ...Unresolved... -
Page 13
... biology. We operate in one business segment - human therapeutics. We market human therapeutic products in the areas of supportive cancer care, nephrology, inflammation and oncology. Our principal products include Aranesp® (darbepoetin alfa), EPOGEN® (Epoetin alfa), Neulasta® (pegfilgrastim... -
Page 14
... purposes. We operate commercial and clinical manufacturing facilities in several locations throughout the United States and in Puerto Rico. Third-party contractors manufacture some or all of certain of our commercial products and/or product candidates. Key Developments The year of 2007 was defined... -
Page 15
...approved label, including targeting higher Hb levels and/or use in non-approved patient populations. As the results of these studies were reported, various regulatory and reimbursement agencies began to review the administration and reimbursement of ESA products resulting in certain key developments... -
Page 16
...data from additional clinical studies. Additionally, the FDA has scheduled an ODAC meeting on March 13, 2008 as part of the FDA's ongoing pharmacovigilance review of ESAs. On October 29, 2007, the European Agency for the Evaluation of Medicinal Products ("EMEA") issued a press release about upcoming... -
Page 17
... on research activities to evaluate ESA therapy in cancer • Working with the FDA to design a large, definitive, well controlled study comparing the safety of ESAs administered to a maximum Hb target of 12 g/dL per the product labeling versus placebo in three major tumor types (non-small cell lung... -
Page 18
...our ESA products. For example, on July 20, 2007, the CMS published revisions to its Claims Monitoring Policy: Erythropoietin/darbepoetin alfa usage for beneficiaries with end stage renal disease ("EMP"), effective January 1, 2008, requiring a 50% reduction in Medicare reimbursement if a patient's Hb... -
Page 19
...license agreement with Takeda Pharmaceutical Company Limited ("Takeda"), which provided them the exclusive rights to develop and commercialize for the Japanese market up to 13 early to mid-stage molecules from our pipeline across a range of therapeutic areas, including oncology and inflammation. The... -
Page 20
... Holdings Company, Limited ("Kirin") (formerly named Kirin Brewery Company, Limited) and Amgen (see "Joint Ventures and Business Relationships - Kirin Holdings Company, Limited"), to manufacture and market darbepoetin alfa in the United States, all European countries, Canada, Australia, New Zealand... -
Page 21
... of cancer by managing tumor growth. We were granted an exclusive license to manufacture and market G-CSF in the United States, Europe, Canada, Australia and New Zealand under a licensing agreement with KA (see "Joint Ventures and Business Relationships - Kirin Holdings Company, Limited"). We market... -
Page 22
...issues which may result in additional patient safety information in the form of a boxed warning that will apply to the ENBREL label as has been the case with other TNF inhibitor agents. ENBREL sales for the years ended December 31, 2007, 2006 and 2005 were $3.2 billion, $2.9 billion and $2.6 billion... -
Page 23
... agencies to conduct further clinical trials to provide additional information on our marketed products' safety and efficacy. These additional trials may include, among other things, studying different doses or schedules of administration that were used in previous studies, use in other patient... -
Page 24
..., well controlled study comparing the safety of ESAs administered to a maximum Hb target of 12 g/dL per the product labeling versus placebo in three major tumor types (NSCLC, breast cancer and advanced CRC). We have agreed on the general study design, and plan to submit a study protocol after... -
Page 25
... have granted J&J a license to commercialize recombinant human erythropoietin as a human therapeutic in the United States in all markets other than dialysis (see "Joint Ventures and Business Relationships - Johnson & Johnson"). Under a co-promotion agreement with Wyeth, Amgen and Wyeth market ENBREL... -
Page 26
... and sales incentive data for Aranesp® from January 1, 2007 through December 31, 2007. CMS publishes the ASPs for products in advance of the quarter in which they go into effect. In the United States, dialysis providers are primarily reimbursed for EPOGEN® by the federal government through the End... -
Page 27
... may go into effect could affect our product sales and related sales growth in the future. For example, the MMA required a report to Congress and a demonstration project with regard to a bundled payment system for dialysis, including separately billable drugs and EPOGEN®. The report to Congress was... -
Page 28
... treatment is the recommended FDA label starting dose, no more than 150 unit ("U")/kilogram ("kg")/three times weekly for Epoetin and 2.25 mcg/kg/weekly for darbepoetin alfa. Equivalent doses may be given over other approved time periods; • Maintenance of ESA therapy is the starting dose if the Hb... -
Page 29
... patients around the world are able to benefit from our future products, we are also seeking partners to assist in the development of selected product candidates in our pipeline in certain countries and/or worldwide. For example, in July 2007, we entered into a collaboration and license agreement... -
Page 30
...table is a selection of certain of our product candidates by phase of development in our therapeutic areas of focus as of February 27, 2008. Additional product candidate (pipeline) information can be found on our website at (http://www.amgen.com). (This website address is not intended to function as... -
Page 31
...additional information about selected product candidates, by therapeutic area, that are in human clinical trials. Oncology We utilize multiple strategies to develop oncology therapeutics. These approaches include developing products that: (i) kill highly proliferative cells, (ii) inhibit cancer cell... -
Page 32
... market (see "Joint Ventures and Business Relationships - Takeda Pharmaceutical Company Limited"). In 2007, enrollment began for a phase 3 study in NSCLC. Additionally, we are conducting head-to-head phase 2 studies of this agent versus bevacizumab in the treatment of metastatic breast cancer... -
Page 33
Inflammation AMG 108 AMG 108 is a fully human monoclonal antibody that targets inhibition of the action of interleukin-1 ("IL-1"), a cytokine known to play a role in the joint destruction associated with rheumatoid arthritis. We recently completed a phase 2 clinical study to investigate the ... -
Page 34
... of type II diabetes. We acquired the rights to this compound in 2007 through our acquisition of Alantos. A phase 2a study is ongoing in this disease setting in collaboration with Servier, which owns the rights outside the United States. General Medicine Aranesp® (darbepoetin alfa) The Trial to... -
Page 35
...by competitors or new information about existing products may result in product replacements or price reductions, even for products protected by patents. Further, the development of new treatment options or standard of care may require less use of our products, particularly in supportive cancer care... -
Page 36
... information on competition related to our principal products and other selected products and product candidates in the therapeutic area(s) in which we market or expect to market them. Supportive cancer care Any products or technologies that are directly or indirectly successful in addressing... -
Page 37
... of anemia. Any products or technologies that are directly or indirectly successful in treating secondary hyperparathyroidism in patients with CKD on dialysis could negatively impact product sales for Sensipar®/Mimpara®. Amgen Marketed Product Competitor Marketed Product Competitor Sensipar... -
Page 38
...2008, we launched Vectibix® in certain EU countries. Amgen Marketed Product Competitor Marketed Product Competitor Vectibix™ - U.S. Vectibix™ - International Product candidates Erbitux® Erbitux® Imclone Systems Incorporated ("Imclone")/ BMS Merck KGaA We are currently studying new product... -
Page 39
...a global supply agreement with Wyeth related to the manufacture, supply, inventory and allocation of bulk supplies of ENBREL. Under this agreement, the Company and Wyeth share the total worldwide bulk supply of ENBREL produced by Amgen's Rhode Island manufacturing facility, BI Pharma's manufacturing... -
Page 40
... Ireland manufacturing operations, certain revisions to our planned manufacturing expansion in Puerto Rico, the accelerated closure of one of our ENBREL commercial bulk manufacturing operations in West Greenwich, Rhode Island and the closure of a clinical manufacturing facility in Thousand Oaks... -
Page 41
...KA develops and commercializes certain of our and Kirin's product rights, which have been transferred to this joint venture. KA has given exclusive licenses to us to manufacture and market: (i) darbepoetin alfa in the United States, all European countries, Canada, Australia, New Zealand, Mexico, all... -
Page 42
... of ENBREL. Under this agreement, the Company and Wyeth share the total worldwide bulk supplies of ENBREL produced by Amgen's Rhode Island manufacturing facility, BI Pharma's manufacturing facility in Germany and Wyeth's manufacturing facility in Ireland. Fresenius Medical Care North America, Inc... -
Page 43
... limiting or costly actions if we or others identify side effects after our products are on the market.", "- Before we commercialize and sell any of our product candidates, we must conduct clinical trials in humans; if we fail to adequately manage these trials we may not be able to sell future... -
Page 44
... the Food and Drug Administration Amendments Act of 2007 (the "FDAAA"), which significantly added to the FDA's authority. Under the FDAAA, if the FDA becomes aware of new safety information after approval of a product, they may require us to conduct further clinical trials to assess a known serious... -
Page 45
...-related problems in a process known as pharmacovigilance. This process includes the collection of adverse drug reaction reports as part of the follow-up on any side effects of a product, and upon assessment, the authorities can decide to demand that the product labels be updated with safety data... -
Page 46
... receive health services grants from the PHS, as well as hospitals that serve a disproportionate share of Medicare and Medicaid beneficiaries. The rebate amount is determined for each quarter based on our reports of the quarter's AMP and best price for each of our products to the CMS. The terms of... -
Page 47
...be required to perform additional clinical trials or change the labeling of our products or take other potentially limiting or costly actions if we or others identify side effects after our products are on the market." and "- Our sales depend on payment and reimbursement from third-party payers, and... -
Page 48
...Chief Operating Officer of the Company. From April 1989 to October 1992, Mr. Sharer was President of the Business Markets Division of MCI Communications Corporation ("MCI"). From February 1984 to March 1989, Mr. Sharer held numerous executive capacities at General Electric Company ("GE"). Mr. Sharer... -
Page 49
... and Corporate Vice President, Quality System. Mr. Robert A. Bradway, age 45, became Executive Vice President and Chief Financial Officer in April 2007. He joined the Company in 2006 as Vice President, Operations Strategy. Previously, Mr. Bradway had an 18 year career at Morgan Stanley in New York... -
Page 50
...the labeling of our products or take other potentially limiting or costly actions if we or others identify side effects after our products are on the market. We and certain of our licensees and partners conduct research, preclinical testing and clinical trials for our product candidates and marketed... -
Page 51
... agencies, the discovery of significant problems with a similar product that implicates an entire class of products, subsequent concerns about the sufficiency of the data or studies underlying the label or changes to the underlying safety/efficacy analysis related to results from clinical trials... -
Page 52
... and Wyeth, or independent investigators) fail to effectively report to regulatory agencies side effects or other safety concerns that occur from their use of our products in clinical trials or studies or from marketed use, regulatory approval may be withdrawn for a product for the therapeutic area... -
Page 53
...approvals, the use, reimbursement and sales of our ESA products. (See "- Before we commercialize and sell any of our product candidates, we must conduct clinical trials in humans; if we fail to adequately manage these trials we may not be able to sell future products and our sales could be adversely... -
Page 54
...the class of ESAs, the reimbursement, use and sales of Aranesp® in Europe could be materially adversely affected. Before we commercialize and sell any of our product candidates, we must conduct clinical trials in humans; if we fail to adequately manage these trials we may not be able to sell future... -
Page 55
... and private insurance plans. Generally, in Europe and other countries outside the United States, the government sponsored healthcare system is the primary payer of healthcare costs of patients. Governments may regulate access to, prices or reimbursement levels of our products to control costs or to... -
Page 56
... may go into effect could affect our product sales and related sales growth in the future. For example, the MMA required a report to Congress and a demonstration project with regard to a bundled payment system for dialysis, including separately billable drugs and EPOGEN®. The report to Congress was... -
Page 57
... policy statement granting, limiting or excluding Medicare coverage or reimbursement for a specific medical item or service. During the initial comment period which ended on April 13, 2007, we submitted comments to CMS which included a detailed and thorough review of the available clinical data... -
Page 58
... treatment is the recommended FDA label starting dose, no more than 150 U/ kg/three times weekly for Epoetin and 2.25 mcg/kg/weekly for darbepoetin alfa. Equivalent doses may be given over other approved time periods; • Maintenance of ESA therapy is the starting dose if the Hb level remains below... -
Page 59
...patent rights. This lawsuit is described in Note 10 "Contingencies - Roche Matters" to the Consolidated Financial Statements. (See "- Our marketed products face substantial competition and other companies may discover, develop, acquire or commercialize products before or more successfully than we do... -
Page 60
... costs and not achieve anticipated cost savings from our recently announced restructuring plan. As a result of recent developments and, in particular the regulatory and reimbursement changes to our marketed ESA products, on August 15, 2007, we announced a plan to restructure our worldwide operations... -
Page 61
...is highly uncertain, and very few R&D projects produce a commercial product. Product candidates or new indications for existing products (collectively, "product candidates") that appear promising in the early phases of development, such as in early human clinical trials, may fail to reach the market... -
Page 62
... limiting or costly actions if we or others identify side effects after our products are on the market." and "- Before we commercialize and sell any of our product candidates, we must conduct clinical trials in humans; if we fail to adequately manage these trials we may not be able to sell future... -
Page 63
... a short period of time to offset reductions in revenue. Further, primarily as a result of the various regulatory and reimbursement developments impacting ESA products, on August 15, 2007, we announced a plan to restructure our worldwide operations in order to improve our cost structure. We have... -
Page 64
... optimal utilization of our manufacturing facilities and the potential to incur excess capacity or impairment charges • changes in our product pricing strategies • changes in wholesaler buying patterns • increased competition from new or existing products • fluctuations in foreign currency... -
Page 65
... effects after our products are on the market.") We currently manufacture our products and product candidates at our manufacturing facilities located in Thousand Oaks and Fremont, California; Boulder and Longmont, Colorado; West Greenwich, Rhode Island; Bothell, Washington and Juncos, Puerto Rico... -
Page 66
...licensure of a new formulation and filling facility at our Puerto Rico site and (iii) expansion of our Fremont, California facility to support future product launches. If regulatory authorities determine that we or our third-party contract manufacturers or third-party service providers have violated... -
Page 67
... and adversely affect our operating results. Among the factors that could affect our actual supply of ENBREL at any time include, without limitation, BI Pharma's and our Rhode Island facility's bulk drug production scheduling. For example, BI Pharma does not produce ENBREL continuously; rather, it... -
Page 68
... to perform additional clinical trials or change the labeling of our products or take other potentially limiting or costly actions if we or others identify side effects after our products are on the market.") Our products may compete against products that have lower prices, equivalent or superior... -
Page 69
...as those of our products and drugs approved for other indications that are used off-label. Large pharmaceutical corporations may have greater clinical, research, regulatory, manufacturing, marketing, financial and human resources than we do. In addition, some of our competitors may have technical or... -
Page 70
... Canada. A management committee comprised of an equal number of representatives from us and Wyeth is responsible for overseeing the marketing and sales of ENBREL including strategic planning, the approval of an annual marketing plan, product pricing and the establishment of a brand team. The brand... -
Page 71
... changes in accounting principles generally accepted in the United States ("GAAP") that may be made affecting accounting for convertible debt securities, some of which could have an adverse impact on our past or future reported financial results. Continual manufacturing process improvement efforts... -
Page 72
Item 1B. UNRESOLVED STAFF COMMENTS None. Item 2. PROPERTIES The following table summarizes our significant properties and their primary functions as of December 31, 2007. For additional information regarding manufacturing initiatives see "Item 1. Business - Manufacturing and Raw Materials." 60 -
Page 73
... certain locations, principally in Thousand Oaks, California; Longmont, Colorado; Louisville, Kentucky; Allentown, Pennsylvania; West Greenwich, Rhode Island; Seattle and Bothell, Washington and Juncos, Puerto Rico, to accommodate future expansion, as required. Also, we purchased land in Ireland in... -
Page 74
... to pay any dividends. The following table sets forth, for the periods indicated, the range of high and low quarterly closing sales prices of the common stock as quoted on The NASDAQ Stock Market: High Low Year ended December 31, 2007 4th Quarter ...3rd Quarter ...2nd Quarter ...1st Quarter ...Year... -
Page 75
... pre-tax value of dividends paid by companies included in these indices and are calculated as of December 31 of each year. The historical stock price performance of the Company's Common Stock shown in the performance graph below is not necessarily indicative of future stock price performance. Amgen... -
Page 76
... in the long-term value of Amgen common stock. Additionally, we believe that it is an effective way of returning cash to our stockholders. A summary of our repurchase activity for the three months ended December 31, 2007 is as follows: Total Number of Shares Purchased as Part of Publicly Announced... -
Page 77
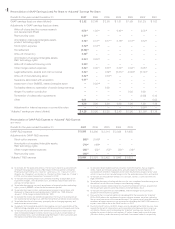
...SELECTED FINANCIAL DATA 2007 Years Ended December 31, 2006 2005 2004 (In millions, except per share data) 2003 Consolidated Statement of Income Data: Revenues: Product sales ...Other revenues ...Total revenues ...Operating expenses(1)(2): Cost of sales (excludes amortization of acquired intangible... -
Page 78
... amortization of acquired product technology rights, primarily ENBREL, related to the Immunex acquisition. Amortization charges, net of tax, for the three years ended December 31, 2007 were $185 million, $200 million and $215 million, respectively. As part of the accounting for the acquisitions of... -
Page 79
... from sales of human therapeutic products in the areas of supportive cancer care, nephrology and inflammation. Our principal products include Aranesp®, EPOGEN®, Neulasta®/NEUPOGEN® and ENBREL all of which are sold in the United States. ENBREL is marketed under a co-promotion agreement with Wyeth... -
Page 80
... future challenges, including the potential for further revisions to product labels and changes to reimbursement. These developments have had and will continue to have a material adverse impact on sales of our ESA marketed products, in particular Aranesp® sales in the U.S. supportive cancer care... -
Page 81
... research efforts in the core areas of oncology, inflammation, bone and metabolic disorders. In order to continue advancing our expanding pipeline of product candidates and to assist in ensuring that patients around the world are able to benefit from our future products, we are also seeking partners... -
Page 82
...-party reimbursement availability and policies, government programs, regulatory developments or guidelines, clinical trial outcomes, clinical practice, pricing strategies, wholesaler and end-user inventory management practices, patient population, fluctuations in foreign currency exchange rates, new... -
Page 83
... for the year ended December 31, 2007 increased 2%. International sales were negatively impacted in Europe by dosing conservatism in the oncology segment and pricing pressures across all ESAs. Through December 31, 2007, biosimilars and other recently introduced marketed products in Europe have not... -
Page 84
... to product information from the EMEA for the class of ESAs, including Aranesp®, in Europe; O • our ability to maintain a competitive segment share and differentiate Aranesp® from current and potential future competition; • adverse events or results from clinical trials or studies performed... -
Page 85
... of our product, regulatory or private healthcare organization medical guidelines and reimbursement practices; • cost containment pressures from the federal government on healthcare providers; and • pricing strategies; any or all of which could have a material adverse impact on future sales of... -
Page 86
...or guidelines relating to the use of our products; cost containment pressures from governments and private insurers on healthcare providers; our ability to minimize distraction due to ESA issues; pricing strategies; patient growth; and development of new treatments for cancer and future chemotherapy... -
Page 87
... our product sales and operating expenses for the years ended December 31, 2007, 2006 and 2005 (dollar amounts in millions): 2007 Change 2006 Change 2005 Product sales ...Operating expenses: Cost of sales (excludes amortization of acquired intangible assets) ...% of product sales ...Research and... -
Page 88
... ENBREL commercial bulk manufacturing operations in connection with the rationalization of our worldwide network of manufacturing facilities. See Note 2, "Restructuring" to the Consolidated Financial Statements for further discussion. In addition, cost of sales for the year ended December 31, 2007... -
Page 89
..., in particular our Global Enterprise Resource Planning ("ERP") system, higher Wyeth profit share expenses related to ENBREL sales and higher legal costs associated with ongoing litigation. SG&A costs for the year ended December 31, 2006 included approximately $120 million in stock option expense... -
Page 90
... the product candidates acquired in the Alantos and Ilypsa acquisitions. Other items (primarily certain restructuring costs in 2007) As discussed in Note 2, "Restructuring" to the Consolidated Financial Statements, on August 15, 2007, we announced a plan to restructure our worldwide operations in... -
Page 91
... for income taxes. See Note 5, "Income taxes" to the Consolidated Financial Statements for further discussion. In August 2007, the FASB exposed for public comment a proposed FSP that would change the method of accounting for convertible debt securities that requires or permits settlement in cash... -
Page 92
...or future reported financial results. For additional discussion on this issue, see "Item 1A. Risk Factors - The accounting method for our convertible debt securities may be subject to change." Financial Condition, Liquidity and Capital Resources The following table summarizes selected financial data... -
Page 93
...Notes and the 2013 Convertible Notes may only be converted (i) during any calendar quarter if the closing price of our common stock exceeds 130% of the respective conversion price per share during a defined period at the end of the previous quarter, (ii) if we make specified distributions to holders... -
Page 94
... facility or commercial paper program as of December 31, 2007. We have a $1.0 billion shelf registration statement (the "$1.0 Billion Shelf") which allows us to issue debt securities, common stock and associated preferred share purchase rights, preferred stock, warrants to purchase debt securities... -
Page 95
... costs associated with implementing our ERP system. Capital expenditures in 2005 primarily related to the Puerto Rico manufacturing expansion which included a new manufacturing plant for the commercial production of Neulasta® and NEUPOGEN® approved by the FDA in September 2005, Thousand Oaks site... -
Page 96
... stock purchase plans provided $277 million, $528 million and $1.1 billion of cash during the years ended December 31, 2007, 2006 and 2005, respectively. Proceeds from the exercise of employee stock options will vary from period to period based upon, among other factors, fluctuations in the market... -
Page 97
... success rates and bulk drug yields achieved; (ii) R&D commitments (including those related to clinical trials) for new and existing products; (iii) capital expenditures and (iv) open purchase orders for the acquisition of goods and services in the ordinary course of business. Our obligation to pay... -
Page 98
... known market events and trends, internal and external historical data and forecasted customer buying patterns. Sales incentives are product-specific and, therefore, for any given year, can be impacted by the mix of products sold. For the years ended December 31, 2007, 2006 and 2005, reductions... -
Page 99
..., working capital and long-term investment requirements necessitate that certain assets associated with these earnings be repatriated to the United States, an additional tax provision and related liability would be required which could materially impact our future effective tax rate. Contingencies... -
Page 100
..., financial position or cash flows. Valuation of acquired intangible assets We have acquired and continue to acquire intangible assets primarily via the acquisition of biotechnology companies. These intangible assets primarily consist of technology associated with human therapeutic products and... -
Page 101
.... On December 31, 2007 and 2006, we were also exposed to price risk on equity securities included in our portfolio of investments, which were acquired primarily for the promotion of business and strategic objectives. These investments are generally in small capitalization stocks in the biotechnology... -
Page 102
... and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of December 31, 2007. Further, management determined that, as of December 31, 2007, there were no changes in our internal control over financial reporting that occurred during the fiscal quarter then... -
Page 103
...inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and reporting. Management assessed the effectiveness of the Company's internal control over financial reporting as of December 31, 2007... -
Page 104
... or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of... -
Page 105
... financial officer, principal accounting officer or controller, and other persons performing similar functions. To view this code of ethics free of charge, please visit our website at www.amgen.com (This website address is not intended to function as a hyperlink, and the information contained... -
Page 106
... either the closing price of the Common Stock on the applicable Purchase Date or the closing price of the Common Stock on the start date of the applicable employee's participation in the plan. These plans were assumed pursuant to the terms of the merger agreement between Amgen and Immunex which was... -
Page 107
... stock are authorized for issuance under the Amgen Limited 2000 U.K. Company Employee Share Option Plan, no shares have been issued under this plan. The Amgen Technology Ireland Irish Tax Approved Share Plan was approved by the Board of Directors on March 7, 2007 and 7,832 shares were purchased... -
Page 108
... shares of common stock in any calendar year. Terms of Discretionary Options. The following is a description of the permissible terms of options granted under the 1999 Plan, other than options awarded to non-employee directors which are described below under the heading "Terms of Non-Discretionary... -
Page 109
... in favor of the Company in accordance with a vesting schedule determined by the Board of Directors. To the extent provided by the terms of a stock bonus or restricted stock purchase agreement, a participant may satisfy any federal, state or local tax withholding obligations relating to the lapsing... -
Page 110
..., litigation, human resources, information services, manufacturing, manufacturing capacity, production, inventory, site development, plant, building or facility development, government relations, product market share, mergers, acquisitions or sales of assets or subsidiaries. The Amgen Limited... -
Page 111
... Technology (Ireland) Limited ("ATI"), the Company's indirectly whollyowned Ireland subsidiary, and approved by the Board of Directors of the Company in March 2007. In general, the Ireland Share Plan permits certain employees of Amgen Limited to buy shares of the Company's common stock during annual... -
Page 112
...each of the three years in the period ended December 31, 2007 ...Notes to Consolidated Financial Statements ...(a)2. Index to Financial Statement Schedules F-1 F-2 F-3 F-4 F-5 F-6 - F-50 The following Schedule is filed as part of this Form 10-K Annual Report: II. Valuation Accounts ... Page number... -
Page 113
... the quarter ended June 30, 2006 on August 9, 2006 and incorporated herein by reference). Registration Rights Agreement, dated as of February 17, 2006, among Amgen Inc. and Merrill Lynch, Pierce, Fenner & Smith Incorporated, Morgan Stanley & Co. Incorporated, Citigroup Global Markets Inc., JPMorgan... -
Page 114
Exhibit No. Description 4.20 Corporate Commercial Paper - Master Note between and among Amgen Inc., as Issuer, Cede & Co., as Nominee of The Depository Trust Company, and Citibank, N.A., as Paying Agent. (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 1998 on May 13, 1998 and ... -
Page 115
... Retirement Plan (As Amended and Restated January 1, 2005). (Filed as an exhibit to Form 10-Q for the quarter ended September 30, 2007 on November 9, 2007 and incorporated herein by reference.) Amgen Inc. Change of Control Severance Plan. (Filed as an exhibit to Form 10-K for the year ended... -
Page 116
...-K for the year ended December 31, 2006 on February 28, 2007 and incorporated herein by reference.) Fourth Amendment to the Amgen Nonqualified Deferred Compensation Plan (As Amended and Restated January 1, 2005). (Filed as an exhibit to Form 10-Q for the quarter ended September 30, 2007 on November... -
Page 117
..., dated June 30, 1988, to Research, Development, Technology Disclosure and License Agreement: GM-CSF dated March 31, 1987, between Kirin Brewery Company, Limited and Amgen Inc. (Filed as an exhibit to Form 8 amending the Quarterly Report on Form 10-Q for the quarter ended June 30, 1988 on August 25... -
Page 118
.... 6 to the Enbrel® Supply Agreement, dated November 27, 2007, among Immunex Corporation, Wyeth (formerly, "American Home Products Corporation") and Boehringer Ingelheim Pharma KG (with certain confidential information deleted therefrom). Agreement Regarding Governance and Commercial Matters, dated... -
Page 119
... Amended and Restated Promotion Agreement, effective as of January 1, 2005, by and among Wyeth, Amgen Inc. and Immunex Corporation (with certain confidential information deleted therefrom). (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 2005 on May 4, 2005 and incorporated herein... -
Page 120
..., between Amgen Inc. and Citigroup Global Markets Inc. (Filed as an exhibit to Form 10-K for the year ended December 31, 2005 on March 10, 2006 and incorporated herein by reference.) Purchase Agreement, dated May 24, 2007, among Amgen Inc., Morgan Stanley & Co. Incorporated, Merrill Lynch, Pierce... -
Page 121
... Securities Exchange Act of 1934, the registrant has duly caused this Annual Report to be signed on its behalf by the undersigned, thereunto duly authorized. AMGEN INC. (Registrant) Date: 02/28/08 By: /s/ ROBERT A. BRADWAY Robert A. Bradway Executive Vice President and Chief Financial Officer 109 -
Page 122
.../S/ KEVIN W. SHARER Kevin W. Sharer Chairman of the Board, Chief Executive Officer and President, and Director (Principal Executive Officer) Executive Vice President and Chief Financial Officer (Principal Financial Officer) Vice President Finance and Chief Accounting Officer (Principal Accounting... -
Page 123
Signature Title Date /S/ J. PAUL REASON J. Paul Reason Director 02/28/08 /S/ LEONARD D. SCHAEFFER Leonard D. Schaeffer Director 02/28/08 111 -
Page 124
... 1991 Equity Incentive Plan, in the Registration Statement (Form S-8 No. 33-47605) pertaining to the Retirement and Savings Plan for Amgen Puerto Rico, Inc., in the Registration Statement (Form S-8 No. 333-44727) pertaining to the Amgen Inc. 1997 Special Non-Officer Equity Incentive Plan, in the... -
Page 125
... to the consolidated financial statements and schedule of Amgen Inc., and the effectiveness of internal control over financial reporting of Amgen Inc., included in this Annual Report (Form 10-K) for the year ended December 31, 2007. /s/ Ernst & Young LLP Los Angeles, California February 25, 2008... -
Page 126
[THIS PAGE INTENTIONALLY LEFT BLANK] -
Page 127
... of operations and cash flows for each of the three years in the period ended December 31, 2007, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken... -
Page 128
... STATEMENTS OF INCOME Years ended December 31, 2007, 2006 and 2005 (In millions, except per share data) 2007 2006 2005 Revenues: Product sales ...Other revenues ...Total revenues ...Operating expenses: Cost of sales (excludes amortization of acquired intangible assets presented below) ...Research... -
Page 129
... AND STOCKHOLDERS' EQUITY Current liabilities: Accounts payable ...Accrued liabilities ...Convertible notes ...Current portion of other long-term debt ...Total current liabilities ...Deferred tax liabilities ...Convertible notes ...Other long-term debt ...Other non-current liabilities ...Commitments... -
Page 130
... INC. CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY Years ended December 31, 2007, 2006 and 2005 (In millions) Number of shares Common stock and additional paid-in capital Accumulated deficit Accumulated other comprehensive income Total Balance at December 31, 2004 ...Comprehensive income: Net... -
Page 131
...) 2007 2006 2005 Cash flows from operating activities: Net income ...$ 3,166 $ 2,950 $ 3,674 Depreciation and amortization ...1,202 963 841 Write-off of acquired in-process research and development ...590 1,231 - Stock-based compensation expense ...263 403 106 Tax benefits related to employee stock... -
Page 132
...sale as defined in Statement of Financial Accounting Standards ("SFAS") No. 115, "Accounting for Certain Investments in Debt and Equity Securities." Accordingly, these investments are recorded at fair value, which is based on quoted market prices. For the years ended December 31, 2007, 2006 and 2005... -
Page 133
... FINANCIAL STATEMENTS (Continued) Amortized cost Gross unrealized gains Gross unrealized losses Estimated fair value December 31, 2006 Type of security: U.S. Treasury securities and obligations of U.S. government agencies ...Corporate debt securities ...Other short-term interest bearing securities... -
Page 134
.... NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued) Inventories Inventories are stated at the lower of cost or market. Cost, which includes amounts related to materials, labor and overhead, is determined in a manner which approximates the first-in, first-out (FIFO) method. During 2007, we wrote... -
Page 135
... included in "Cost of sales" and "Selling, general and administrative" expense in the Consolidated Statements of Income. Acquired product technology rights relate to the identifiable intangible assets acquired in connection with the Immunex Corporation ("Immunex") acquisition in July 2002... -
Page 136
... associated with R&D personnel, overhead and occupancy, clinical trials and related clinical manufacturing, including contract services and other outside costs, process development, quality assurance, information systems and amortization of acquired technology used in R&D with alternative future... -
Page 137
...States and Canada are reserved to Wyeth. We also have a global supply agreement with Wyeth related to the manufacture, supply, inventory and allocation of bulk supplies of ENBREL. Advertising costs are expensed as incurred. For the years ended December 31, 2007, 2006 and 2005, advertising costs were... -
Page 138
...97 $ 2.93 For the years ended December 31, 2007, 2006 and 2005, there were employee stock options, calculated on a weighted average basis, to purchase 48 million, 13 million and 16 million shares, respectively, with exercise prices greater than the average market prices of common stock that are not... -
Page 139
... years ended December 31, 2007, 2006 and 2005, gains and losses on these interest rate swap agreements were not material and were fully offset by the losses and gains on the hedged debt instruments through current earnings. Recent Accounting Pronouncements In December 2007, the Financial Accounting... -
Page 140
..., these decisions included certain revisions to and the subsequent indefinite postponement of our planned Ireland manufacturing operations, certain revisions to our planned manufacturing expansion in Puerto Rico and the closure of a clinical manufacturing facility in Thousand Oaks, California. F-14 -
Page 141
... with our co-promotion agreement with Wyeth. Such amounts are recorded as a reduction of SG&A expenses. In addition during the year ended December 31, 2007, we accrued $119 million, primarily related to loss accruals for leases for certain R&D facilities that will not be used in our operations. Such... -
Page 142
... Statements of Income for the years ended December 31, 2007, 2006 and 2005 (in millions): 2007 2006 2005 Stock options ...Restricted stock ...Performance units ...Total stock-based compensation expense, pre-tax ...Tax benefit from stock-based compensation expense ...Total stock-based compensation... -
Page 143
... volatility in publicly traded instruments associated with Amgen's common stock during the period the option is granted. We believe implied volatility in these instruments is more indicative of expected future volatility than the historical volatility in the price of our common stock. Upon the... -
Page 144
... INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued) Stock option information with respect to our stock-based compensation plans during the three years ended December 31, 2007 is as follows: Options (in millions) Weighted-average exercise price Weighted-average remaining contractual life... -
Page 145
... restricted stock awards subject to graded vesting that were issued after January 1, 2006, we recognize compensation cost on a straight-line basis over the service period for the entire award. Performance award program In 2004, 2005 and 2006 certain management-level employees received annual grants... -
Page 146
... TO CONSOLIDATED FINANCIAL STATEMENTS (Continued) 4. Related party transactions We own a 50% interest in KA, a corporation formed in 1984 with Kirin Holdings Company, Limited ("Kirin") (formerly named Kirin Brewery Company, Limited) for the development and commercialization of certain products based... -
Page 147
... December 31, 2007 2006 Deferred tax assets: Intercompany inventory related items ...Expense accruals ...Acquired net operating loss and credit carryforwards ...Expenses capitalized for tax ...Convertible debt ...Stock-based compensation ...Other ...Total deferred tax assets ...Valuation allowance... -
Page 148
... of this examination primarily related to transfer pricing tax positions. Our closing agreement with the IRS also covers certain transfer pricing issues for the years ended December 31, 2005 and 2006; however, these years have not been effectively settled. As of December 31, 2007, we believe that it... -
Page 149
... provided a temporary incentive to repatriate undistributed foreign earnings. One provision of the American Jobs Creation Act reduced the effective tax rate by providing an 85% dividends-received deduction for certain dividends from controlled foreign corporations. In the fourth quarter of 2005, we... -
Page 150
... rates will be adjusted if we make specified types of distributions or enter into certain other transactions in respect to our common stock. The 2011 Convertible Notes and the 2013 Convertible Notes may only be converted: (i) during any calendar quarter if the closing price of our common stock... -
Page 151
... rata portion, $51 million, of deferred financing and related costs were immediately charged to interest expense. Holders of 2032 Modified Convertible Notes may convert each of their notes based on a conversion rate of 8.8601 shares of common stock. The conversion price per share of the convertible... -
Page 152
... of other debt securities. Shelf registration statements and other facilities In 2007, we established a $2.5 billion unsecured revolving credit facility to be used for general corporate purposes, including commercial paper support, which matures in November 2012 and replaces our prior $1.0 billion... -
Page 153
... months ended June 30, 2007 excludes 2.5 million shares received in July 2007 in connection with the final settlement of a block trade entered into in May 2007, which is discussed in Note 6, "Financing Arrangements" above. As of December 31, 2007, $6.4 billion was available for stock repurchases... -
Page 154
... - Acquired in-process research and development"). The results of Alantos' operations have been included in the consolidated financial statements commencing July 16, 2007. Pro forma results of operations for the year ended December 31, 2007 assuming the acquisition of Alantos had taken place at... -
Page 155
... - Acquired in-process research and development"). The results of Ilypsa's operations have been included in the consolidated financial statements commencing July 18, 2007. Pro forma results of operations for the year ended December 31, 2007 assuming the acquisition of Ilypsa had taken place at... -
Page 156
... - Acquired in-process research and development"). The results of Abgenix's operations have been included in the consolidated financial statements commencing April 1, 2006. Pro forma results of operations for the year ended December 31, 2006 assuming the acquisition of Abgenix had taken place at... -
Page 157
... commitments for the purchase of ENBREL and reflect certain estimates such as production run success rates and bulk drug yields achieved. Amounts purchased under contractual inventory commitments from third-party contract manufacturers for the years ended December 31, 2007, 2006 and 2005 were $153... -
Page 158
AMGEN INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued) product... the effect of two...trial... 6, 2005, the... court found that claims...grant injunctive relief. The Massachusetts District Court set a schedule for briefs on whether the record should be opened for further evidence. On July 17, 2007... -
Page 159
...CONSOLIDATED FINANCIAL STATEMENTS (Continued) Average Wholesale Price Litigation Amgen and Immunex are named as defendants, either separately or together, in numerous civil actions broadly alleging that they, together with many other pharmaceutical manufacturers, reported prices for certain products... -
Page 160
.... Ven-A-Care of the Florida Keys, Inc. v. Abbott Laboratories, Inc., et al.). Immunex, and not Amgen, was a defendant in the California AG complaint until Immunex was dismissed from the case on January 17, 2007, following a settlement agreement entered into between the parties, executed on December... -
Page 161
... have filed a joint motion to dismiss and a hearing on the motions to dismiss is set for March 13, 2008. County of Erie v. Abbott Laboratories, Inc., et al. This case was filed against Amgen and Immunex on March 8, 2005, in the Supreme Court of New York, Erie County. The complaint alleges that all... -
Page 162
... in the Superior Court of New Jersey, Monmouth County. The complaint alleges that Amgen and Immunex, together with many other pharmaceutical manufacturers, reported prices for certain products in a manner that allegedly inflated reimbursement under the New Jersey state Medicaid program. Defendants... -
Page 163
... New York, Oswego County. Immunex Governmental Investigations According to press reports, many pharmaceutical companies are under investigation by the U.S. Department of Justice, the U.S. Department of Health and Human Services, and/or state agencies related to the pricing of their products. Immunex... -
Page 164
...15 U.S.C. Sections 1 and 2. The complaint sought a preliminary injunction to enjoin Amgen from offering discounts to oncology clinics on its G-CSF products (NEUPOGEN® and Neulasta®) and Aranesp® if customers purchased certain amounts of both types of products. Ortho Biotech also seeks a permanent... -
Page 165
...INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued) On April 11, 2006, Roche filed motions to dismiss the lawsuit arguing a lack of subject matter jurisdiction and lack of personal jurisdiction over F. Hoffmann-La Roche Ltd. and Roche Diagnostics GmbH. Amgen filed its response to the motions... -
Page 166
...pending summary judgment motions, except Amgen's motion for summary judgment relating to Roche's antitrust allegations, which the Massachusetts District Court has taken under advisement. During the period starting September 4, 2007 and ending October 18, 2007, Amgen's patent infringement claims were... -
Page 167
...Inc., et al. v. Ariad Pharmaceuticals, Inc. On April 20, 2006, Amgen, Immunex, Amgen USA Inc., Amgen Manufacturing, Limited and Immunex Rhode Island Corporation (the "Amgen Entities") filed a complaint against Ariad Pharmaceuticals, Inc. ("Ariad") in the United States District Court for the district... -
Page 168
... Wyeth and Ariad filed a stipulated dismissal without prejudice and the Delaware District Court granted the motion on December 12, 2007. Human Genome Sciences Litigation On August 30, 2007, Human Genome Sciences ("HGS") filed an action under 35 U.S.C. §146 against Amgen Inc. and Immunex Corporation... -
Page 169
... artificially inflating the prices of Amgen's publicly traded securities and (iii) causing plaintiff and other members of the Class to purchase Amgen publicly traded securities at inflated prices. The complaint also makes off-label marketing allegations that, throughout the class period, the Federal... -
Page 170
... employees who participated in the Amgen Retirement and Savings Manufacturing Plan and the Amgen Savings Plan of the alleged off-label promotion of both Aranesp® and EPOGEN® while a number of studies allegedly demonstrated safety concerns in patients using ESAs. On February 4, 2008, the California... -
Page 171
...of ESA studies. On May 10, 2007, Amgen received a subpoena from the Attorney General of the State of New York seeking documents related to Amgen's promotional activities, sales and marketing activities, medical education, clinical studies, pricing and contracting, license and distribution agreements... -
Page 172
... INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued) On January 14, 2008, Amgen received a subpoena from the New Jersey Attorney General's Office for production of documents relating to one of its products. Amgen intends to cooperate fully in responding to the subpoena. On January 14, 2008... -
Page 173
... had three large wholesaler customers each accounting for more than 10% of total revenues for the years ended December 31, 2007, 2006 and 2005. On a combined basis, these distributors accounted for 57% and 71% of total worldwide gross revenues and U.S. gross product sales, respectively, for 2007, as... -
Page 174
...TO CONSOLIDATED FINANCIAL STATEMENTS (Continued) 12. Accrued liabilities Accrued liabilities consisted of the following (in millions): December 31, 2007 2006 Sales incentives ...Employee compensation and benefits ...Clinical development costs ...Accrued royalties ...Income taxes ...Other ... $1,064... -
Page 175
... asset impairments associated with our restructuring plan; and income tax benefit of $92 million recognized as the result of resolving certain non-routine transfer pricing issues with the Internal Revenue Service for prior periods. pro-rata portion of the deferred financing and related costs of $51... -
Page 176
...Consolidated Statement of Income: a. b. benefit of $60 million from favorable tax audit settlements; and charge of $49 million ($31 million, net of tax) related to the impairment of a non-ENBREL related intangible asset previously acquired in the Immunex acquisition. (7) In the second quarter 2006... -
Page 177
SCHEDULE II AMGEN INC. VALUATION ACCOUNTS Years ended December 31, 2007, 2006 and 2005 (In millions) Balance at beginning of period Additions charged to costs and expenses Other additions Balance at end of period Deductions Year ended December 31, 2007: Allowance for doubtful accounts ...Year ... -
Page 178
[THIS PAGE INTENTIONALLY LEFT BLANK] -
Page 179
Amgen Mission To serve patients Amgen Values Be science-based Compete intensely and win Create value for patients, staff and stockholders Be ethical Trust and respect each other Ensure quality Work in teams Collaborate, communicate and be accountable -
Page 180
Amgen Inc. One Amgen Center Drive Thousand Oaks, CA 91320-1799 www.amgen.com © 2008 Amgen Inc. All rights reserved. MC39651