Medtronic 2012 Annual Report Download
Download and view the complete annual report
Please find the complete 2012 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages
listed below, or by using the keyword search tool below to find specific information within the annual report.
-

1
-

2
-

3
-
4
-

5
-

6
-

7
-

8
-

9
-

10
-

11
-

12
-

13
-

14
-

15
-

16
-

17
-

18
-

19
-

20
-

21
-

22
-

23
-

24
-

25
-

26
-

27
-

28
-

29
-

30
-

31
-

32
-

33
-

34
-

35
-

36
-

37
-

38
-

39
-

40
-

41
-

42
-

43
-

44
-

45
-

46
-

47
-

48
-

49
-

50
-

51
-

52
-

53
-

54
-

55
-

56
-

57
-

58
-

59
-

60
-

61
-

62
-

63
-

64
-

65
-

66
-

67
-

68
-

69
-

70
-

71
-

72
-

73
-

74
-

75
-

76
-

77
-

78
-

79
-

80
-

81
-

82
-

83
-

84
-

85
-

86
-

87
-

88
-

89
-

90
-

91
-

92
-

93
-

94
-

95
-

96
-

97
-

98
-

99
-

100
-

101
-

102
-

103
-

104
-

105
-

106
-

107
-

108
-

109
-

110
-

111
-

112
-

113
-

114
-

115
-

116
-

117
-

118
-

119
-

120
-

121
-

122
-

123
-

124
-

125
-

126
-

127
-

128
-

129
-

130
-

131
-

132
-

133
-

134
-

135
-

136
-

137
-

138
-

139
-

140
-

141
-

142
-

143
-

144
-

145
-

146
-

147
-

148
-

149
-

150
-

151
-

152
Table of contents
-
Page 1
-
Page 2
-
Page 3
-
Page 4
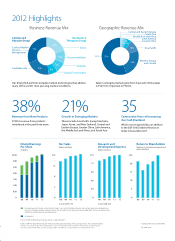
... Therapies Group
31%
Cardiac Rhythm Disease Management
Spinal
Asia Paciï¬c
11%
Neuromodulation
U.S.
55% 25%
9%
Western Europe and Canada
Diabetes Surgical Technologies
CardioVascular
21%
8%
Our diversified portfolio includes medical technologies that address many of the world...
-
Page 5
-
Page 6
-
Page 7
-
Page 8
-
Page 9
-
Page 10
-
Page 11
-
Page 12
... Capital Investor Director since 2000
Rob ten Hoedt
Medtronic Corporate Leadership
Omar Ishrak
Chairman and Chief Executive Officer
Kendall J. Powell
Chairman and Chief Executive Officer, General Mills, Inc. Director since 2007
Senior Vice President and President, EMEA and Canada
Business...
-
Page 13
... (Address of principal executive offices) (Zip Code) Registrant's telephone number, including area code: (763) 514-4000 Securities registered pursuant to Section 12(b) of the Act: Title of each class Common stock, par value $0.10 per share Name of each exchange on which registered New York Stock...
-
Page 14
-
Page 15
... with Accountants on Accounting and Financial Disclosure ...Controls and Procedures ...Other Information ...PART III Directors, Executive Ofï¬cers, and Corporate Governance ...Executive Compensation ...Security Ownership of Certain Beneï¬cial Owners and Management and Related Shareholder Matters...
-
Page 16
Investor Information Annual Meeting and Record Dates Medtronic, Inc.'s (Medtronic or the Company) Annual Meeting of Shareholders will be held on Thursday, August 23, 2012 at 10:30 a.m., Central Daylight Time at the Company's World Headquarters, 710 Medtronic Parkway, Minneapolis (Fridley), Minnesota...
-
Page 17
... Stent Graft System, Enlite™, HeartRescueSM, INFUSE® Bone Graft, InterStim® Therapy, MAST®, MasterGraft®, Melody® Transcatheter Pulmonary Valve, MiniMed® Paradigm® Veo™ System (VEO), MiniMed® Revel™ Systems (Revel), NIM® 3.0 Nerve Monitoring System, O-arm® 3.1.2, O-arm® Imaging...
-
Page 18
...alleviate pain, restore health, and extend life." We currently function in two operating segments that manufacture and sell device-based medical therapies. Our operating segments are as follows:
Fiscal Year 2012
•
Cardiac and Vascular Group Cardiac Rhythm Disease Management (CRDM) CardioVascular...
-
Page 19
CARDIAC AND VASCULAR GROUP Cardiac Rhythm Disease Management CRDM develops, manufactures, and markets products for the diagnosis, treatment, and management of heart rhythm disorders and heart failure, including implantable devices, leads and delivery systems, products for the treatment of atrial ï¬...
-
Page 20
...the obstructed vessel. Balloon angioplasty can be followed up with a coronary stent, a support device which works as scaffolding to keep the vessel open following the intervention. Our PCI stent products include our Integrity, Driver, and Micro-Driver bare metal stent systems as well as our Resolute...
-
Page 21
... to create ablation lines during cardiac surgery. The charts below set forth net sales of our CardioVascular products as a percentage of our total net sales for each of the last three ï¬scal years:
Fiscal Year 2012
(dollars in millions) CardioVascular $3,475
Fiscal Year 2011
(dollars in millions...
-
Page 22
... Structural Heart business are Edwards LifeSciences Corporation, St. Jude, Terumo Medical Corporation, and Sorin Group. RESTORATIVE THERAPIES GROUP Spinal Our Spinal business develops, manufactures, and markets a comprehensive line of medical devices and implants used in the treatment of the spine...
-
Page 23
...fecal, and gastroenterological disorders. The following are the principal products offered by our Neuromodulation business: Neurostimulators for Chronic Pain. Spinal cord stimulation uses a surgically implanted medical device, similar to a cardiac pacemaker, to deliver mild electrical signals to the...
-
Page 24
.... Diabetes Our Diabetes business develops, manufactures, and markets advanced, integrated diabetes management solutions that include insulin pump therapy, continuous glucose monitoring systems, and therapy management software. The following are the principal products offered by our Diabetes business...
-
Page 25
... planning during precision cranial, spinal, sinus, and orthopedic surgeries. In August 2011, we completed the acquisition of two privately-held companies, PEAK Surgical, Inc. (PEAK) and Salient Surgical Technologies, Inc. (Salient), that are focused on advanced energy devices. PEAK is a recognized...
-
Page 26
... for existing products, as well as less invasive and new technologies to address unmet patient needs and to help reduce patient care costs and length of hospital stays. We have not engaged in signiï¬cant customer or government-sponsored research. During ï¬scal years 2012, 2011, and 2010, we spent...
-
Page 27
... Fiscal Year 2012 On August 31, 2011, we acquired Salient. Salient develops and markets devices for haemostatic sealing of soft tissue and bone incorporating advanced energy technology. Salient's devices are used in a variety of surgical procedures including orthopedic surgery, spine, open abdominal...
-
Page 28
... and sales strategy is focused on rapid, cost-effective delivery of high-quality products to a diverse group of customers worldwide - including physicians, hospitals, other medical institutions, and group purchasing organizations. To achieve this objective, we organize our marketing and sales teams...
-
Page 29
.... Working Capital Practices Our goal is to carry sufï¬cient levels of inventory to ensure adequate supply of raw materials from suppliers and meet the product delivery needs of our customers. We also provide payment terms to customers in the normal course of business and rights to return product...
-
Page 30
... the Inspector General of the Department of Health and Human Services, the Department of Justice (DOJ), and various state Attorneys General) monitor the manner in which we promote and advertise our products. Although surgeons are permitted to use their medical judgment to employ medical devices for...
-
Page 31
... generally in the form of their ministries or departments of health, oversee the clinical research for medical devices and are responsible for market surveillance of products once they are placed on the market. We are required to report device failures and injuries potentially related to product use...
-
Page 32
...business, including the U.S. These changes are causing the marketplace to put increased emphasis on the delivery of more cost-effective medical devices and therapies. Government programs, including Medicare and Medicaid, private health care insurance, and managed-care plans have attempted to control...
-
Page 33
..., including medical and dental costs, physical loss to property, business interruptions, workers' compensation, comprehensive general, director and ofï¬cer, and product liability. Decisions to self-insure are based on comparisons between the price, availability, and value of insurance coverage. We...
-
Page 34
... and was promoted to Vice President of Finance for Medtronic Europe in 1992, until being named as Corporate Controller in 1994. Mr. Ellis is a member of the board of directors of The Toro Company and past chairman of the American Heart Association. D. Cameron Findlay, age 52, has been Senior Vice...
-
Page 35
...product technology, product quality, breadth of product lines, product services, customer support, price, and reimbursement approval from health care insurance providers.
Major shifts in industry market share have occurred in connection with product problems, physician advisories, and safety alerts...
-
Page 36
... harm to the public health. The U.S. FDA may also impose operating restrictions on a company-wide basis, enjoin and/or restrain certain conduct resulting in violations of applicable law pertaining to medical devices, and assess civil or criminal penalties against our ofï¬cers, employees, or us. The...
-
Page 37
... affect our reputation and business operations. Quality problems with our processes, goods, and services could harm our reputation for producing high-quality products and erode our competitive advantage, sales, and market share. Quality is extremely important to us and our customers due to the...
-
Page 38
... services due to pricing pressure experienced by our customers from managed care organizations and other third-party payers, increased market power of our customers as the medical device industry consolidates, and increased competition among medical engineering and manufacturing services providers...
-
Page 39
... exchange rates may reduce the reported value of our foreign currency revenues, net of expenses, and cash ï¬,ows. We cannot predict changes in currency exchange rates, the impact of exchange rate changes, nor the degree to which we will be able to manage the impact of currency exchange rate changes...
-
Page 40
... to whom our customers supply medical devices, rely on third-party payers, including government programs and private health insurance plans, to reimburse some or all of the cost of the procedures in which medical devices that incorporate components we manufacture or assemble are used. The continuing...
-
Page 41
... our strategy to develop and identify new products and technologies, we have made several acquisitions in recent years and may make additional acquisitions in the future. Our integration of the operations of acquired businesses requires signiï¬cant efforts, including the coordination of information...
-
Page 42
...have difï¬culty attracting new customers, have problems in determining product cost estimates and establishing appropriate pricing, have difï¬culty preventing, detecting, and controlling fraud, have disputes with customers, physicians, and other health care professionals, have regulatory sanctions...
-
Page 43
...Common Equity, Related Shareholder Matters, and Issuer Purchases of Equity Securities The Company's common stock is listed on the New York Stock Exchange under the symbol "MDT." In June 2009 and June 2011, the Company's Board of Directors authorized the repurchase of 60 million and 75 million shares...
-
Page 44
...the cumulative total shareholder return on Medtronic's common stock with the cumulative total shareholder return on the Standard & Poor's (S&P) 500 Index and the S&P 500 Health Care Equipment Index for the last ï¬ve ï¬scal years. The graph assumes that $100 was invested at market close on April 27...
-
Page 45
Item 6. Selected Financial Data
Fiscal Year 2012 2011 2010 2009 2008
(in millions, except per share data and additional information)
Operating Results for the Fiscal Year: Net sales ...Cost of products sold ...Gross margin percentage ...Research and development expense ...Selling, general, and ...
-
Page 46
... of the Cardiac Rhythm Disease Management (CRDM) and CardioVascular businesses) and the Restorative Therapies Group (composed of the Spinal, Neuromodulation, Diabetes, and Surgical Technologies businesses). Net earnings (including Physio-Control) for the ï¬scal year ended April 27, 2012 were $3.617...
-
Page 47
... Charges, Net, and Acquisition-Related Items" section of this management's discussion and analysis.
Net Sales Fiscal Year 2012 2011 _____ _____
(dollars in millions) _____
% Change _____
Cardiac and Vascular Group ...Restorative Therapies Group ...Total Net Sales ...
8,482 $ 8,119 7,702...
-
Page 48
obligations, sales returns and discounts, stock-based compensation, valuation of equity and debt securities, and income tax reserves are updated as appropriate, which in most cases is quarterly. We base our estimates on historical experience, actuarial valuations, or various assumptions that are ...
-
Page 49
... on our tax return but has not yet been recognized as an expense in our consolidated statements of earnings. The Company's overall tax rate from continuing operations including the tax impact of restructuring charges, net, certain litigation charges, net, and acquisition-related items has resulted...
-
Page 50
... adjustment related to Physio-Control and an increase in the net assets sold. Beginning in the third quarter of ï¬scal year 2012, the assets and liabilities of this business met the accounting criteria to be classiï¬ed as held for sale and have been aggregated and reported on separate lines in...
-
Page 51
... Fiscal Year 2012 2011 % Change _____ 2011 2010 % Change
(dollars in millions) _____
Deï¬brillation Systems Pacing Systems AF and Other CARDIAC RHYTHM DISEASE MANAGEMENT Coronary Structural Heart Endovascular and Peripheral CARDIOVASCULAR TOTAL CARDIAC AND VASCULAR GROUP Core Spinal Biologics...
-
Page 52
... Stent Graft System continues to perform well in the U.S. Additionally, the acquisitions and integration of Ardian and ATS Medical, which were acquired in January 2011 and August 2010, respectively, contributed to ï¬scal year 2012's overall net sales growth. The Cardiac and Vascular Group net sales...
-
Page 53
... outside the U.S. in our Coronary, Structural Heart, and Endovascular and Peripheral businesses. The primary contributors to net sales growth were driven by new product introductions including the Resolute drug-eluting stent and our Integrity bare metal stent within Coronary, the Endurant Abdominal...
-
Page 54
... the Spinal, Neuromodulation, Diabetes, and Surgical Technologies businesses. Products in the Restorative Therapies Group include products for various areas of the spine, bone graft substitutes, biologic products, implantable neurostimulation therapies and drug delivery devices for the treatment of...
-
Page 55
...of ENT, Power Systems, and Navigation product lines, as well as growth across capital equipment, disposables, and service. Additionally, net sales for ï¬scal year 2012 were positively impacted by the August 2011 acquisitions of Salient and PEAK. The Restorative Therapies Group net sales for ï¬scal...
-
Page 56
... issues regarding AMPLIFY. Spinal sales growth was negatively impacted from the June 2011 articles in The Spine Journal and by inquiries from governmental authorities, relating to our INFUSE bone graft product. The Spine Journal articles suggested that some physicians' peer-reviewed studies may have...
-
Page 57
... of the Surgical Technologies NIM 3.0 Nerve Monitoring System.
•
•
•
•
• •
Costs and Expenses The following is a summary of major costs and expenses as a percent of net sales:
Fiscal Year 2012 2011 2010
Cost of products sold ...Research and development ...Selling, general, and...
-
Page 58
... businesses, as well as in new areas, through acquisitions, licensing agreements, alliances, and certain strategic equity investments. Selling, General, and Administrative Fiscal year 2012 selling, general, and administrative expense was $5.623 billion, which as a percent of net sales decreased...
-
Page 59
... of a product line within the CardioVascular business. In the fourth quarter of ï¬scal year 2012, the Company recorded a $31 million reversal of excess restructuring reserves related to the ï¬scal year 2011 initiative. This reversal was primarily a result of certain employees identiï¬ed...
-
Page 60
... recorded certain litigation charges, net of $90 million related to the agreement in principle to settle the federal securities class action initiated by the Minneapolis Fireï¬ghters' Relief Association in December 2008. During the fourth quarter of ï¬scal year 2012, Medtronic reached a settlement...
-
Page 61
... Annual Report on Form 10-K. During ï¬scal year 2012, we reclassiï¬ed $12 million of Physio-Control divestiture-related costs previously recorded in acquisition-related items within continuing operations on the consolidated statements of earnings in the ï¬rst and second quarters of ï¬scal year...
-
Page 62
... Affordability Reconciliation Act of 2010 impose signiï¬cant new taxes on medical device makers in the form of a 2.3 percent excise tax on U.S. medical device sales, with certain exemptions, beginning in January 2013. We currently estimate that our ï¬scal year 2013 excise tax fee (impacting only...
-
Page 63
... federal, state, and foreign income tax audits, ï¬nalization of certain tax returns, and changes to uncertain tax position reserves. In addition, during the fourth quarter of ï¬scal year 2012, we entered into a sale-leaseback agreement that was recorded as a capital lease and as a result of the...
-
Page 64
... for additional information. Liquidity and Capital Resources
(dollars in millions) _____ Fiscal Year _____ __ _ 2012 2011 _____ _____
Working capital(1) ...Current ratio* ...Cash, cash equivalents, and short-term investments ...Long-term investments in debt, marketable equity and trading securities...
-
Page 65
... investments, as well as our syndicated credit facility and related commercial paper program discussed above. Standard & Poor's Ratings Services short-term debt ratings remain unchanged at A-1+ as compared to the ï¬scal year ended April 29, 2011. Our net cash position in ï¬scal year 2012 increased...
-
Page 66
... Annual Report on Form 10-K for additional information regarding fair value measurements. Summary of Cash Flows
(in millions) _____ Fiscal Year _____ 2012 2011 2010
Cash provided by (used in): Operating activities ...Investing activities ...Financing activities ...Effect of exchange rate changes...
-
Page 67
... in "Item 8. Financial Statements and Supplementary Data" in this Annual Report on Form 10-K for additional information regarding contingent consideration. In the normal course of business, we periodically enter into agreements that require us to indemnify customers or suppliers for speciï¬c risks...
-
Page 68
... 8. Financial Statements and Supplementary Data" in this Annual Report on Form 10-K for additional information regarding the interest rate swap agreements. (7) Capital lease obligations include the $165 million sale-leaseback agreement entered into in the fourth quarter of ï¬scal year 2012 whereby...
-
Page 69
... due 2042. Interest on each series of 2012 Senior Notes is payable semi-annually, on March 15 and September 15 of each year, commencing September 15, 2012. We used the net proceeds from the sale of the 2012 Senior Notes for working capital and general corporate purposes. In April 2006, we issued...
-
Page 70
.... Acquisitions Fiscal Year 2012 On August 31, 2011, we acquired Salient. Salient develops and markets devices for haemostatic sealing of soft tissue and bone incorporating advanced energy technology. Salient's devices are used in a variety of surgical procedures including orthopedic surgery, spine...
-
Page 71
..., which was recorded within acquisition-related items in the consolidated statement of earnings in the second quarter of ï¬scal year 2012. Net of this ownership position, the transaction value was approximately $96 million. Fiscal Year 2011 On January 13, 2011, we acquired privately-held Ardian. We...
-
Page 72
...year 2012. Outside the U.S., net sales growth was led by strong double-digit growth in CardioVascular, Spinal, Diabetes, and Surgical Technologies. Within the Cardiac and Vascular Group, net sales were led by increased sales of our Resolute Integrity drug-eluting coronary stent, transcatheter valves...
-
Page 73
...Factors" in this Annual Report on Form 10-K, as well as those related to competition in the medical device industry, reduction or interruption in our supply, quality problems, liquidity, decreasing prices, adverse regulatory action, litigation success, self-insurance, health care policy changes, and...
-
Page 74
... Capital Resources" section of "Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations" in this Annual Report on Form 10-K. For additional discussion of market risk, see Notes 6 and 10 to the consolidated ï¬nancial statements in "Item 8. Financial Statements...
-
Page 75
...Reports of Management Management's Report on the Financial Statements The management of Medtronic, Inc. is responsible for the integrity of the ï¬nancial information presented in this Annual Report on Form 10-K. The consolidated ï¬nancial statements have been prepared in accordance with accounting...
-
Page 76
... Management's Annual Report on Internal Control over Financial Reporting. Our responsibility is to express opinions on these ï¬nancial statements, on the ï¬nancial statement schedule, and on the Company's internal control over ï¬nancial reporting based on our integrated audits. We conducted...
-
Page 77
Medtronic, Inc. Consolidated Statements of Earnings
Fiscal Year _____ 2012 2011 2010 in millions, except per share data) _____
Net sales ...Costs and expenses: Cost of products sold ...Research and development expense ...Selling, general, and administrative expense ...Restructuring charges, net ...
-
Page 78
... compensation and retirement beneï¬ts ...Long-term accrued income taxes ...Long-term deferred tax liabilities, net ...Other long-term liabilities ...Total liabilities ...Commitments and contingencies (Notes 5, 16 and 17) Shareholders' equity: Preferred stock- par value $1.00; 2.5 million shares...
-
Page 79
...Total comprehensive income ...Dividends to shareholders ...Issuance of common stock under stock purchase and award plans ...Repurchase of common stock ...Tax beneï¬t/(deï¬cit) from exercise of stock-based awards ...Stock-based compensation ...Balance as of April 29, 2011 ...Net earnings ...Other...
-
Page 80
... Flows
Fiscal Year 2012 2011 2010 in millions) _____
Operating Activities: Net earnings ...Adjustments to reconcile net earnings to net cash provided by operating activities: Depreciation and amortization ...Amortization of discount on senior convertible notes ...Gain on sale of Physio-Control...
-
Page 81
... technology - alleviating pain, restoring health, and extending life for millions of people around the world. The Company provides innovative products and therapies for use by medical professionals to meet the health care needs of their patients. Primary products include those for cardiac rhythm...
-
Page 82
... of the gains and losses recognized on equity and other securities. Accounts Receivable The Company grants credit to customers in the normal course of business, but generally does not require collateral or any other security to support its receivables. The Company maintains an allowance for doubtful...
-
Page 83
... these therapies. Contingent Consideration During ï¬scal year 2010, as mentioned above, the Company adopted authoritative guidance related to business combinations. Under this guidance, the Company must recognize contingent purchase price consideration at fair value at the acquisition date. Prior...
-
Page 84
... the Company's policy to self-insure the vast majority of its insurable risks including medical and dental costs, disability coverage, physical loss to property, business interruptions, workers' compensation, comprehensive general, director and ofï¬cer, and product liability. Insurance coverage is...
-
Page 85
... on equity securities, and the Puerto Rico excise tax. Stock-Based Compensation The Company's compensation programs include share-based payments. All awards under share-based payment programs are accounted for at fair value and these fair values are generally amortized on a straight-line basis...
-
Page 86
... 2012, 2011, and 2010, respectively. The tax expense (beneï¬t) on the unrealized gain/(loss) on investments in ï¬scal years 2012, 2011, and 2010 was $(38) million, $130 million, and $35 million, respectively. During ï¬scal year 2011, the Company received shares in the form of a dividend related...
-
Page 87
... stock-based awards granted under stock-based compensation plans and shares committed to be purchased under the employee stock purchase plan. The table below sets forth the computation of basic and diluted earnings per share:
(in millions, except per _____ share data Fiscal Year 2012 _ 2011 _ 2010...
-
Page 88
... quarter of ï¬scal year 2012, Medtronic reached a settlement agreement to resolve all of these class claims for $85 million and incurred $5 million in additional litigation fees as a result of the agreement. Refer to Note 17 for additional information. During ï¬scal year 2011, the Company recorded...
-
Page 89
... operations for ï¬scal years 2012, 2011, and 2010:
(in millions Fiscal Year 2012 _ 2011 _ 2010
Discontinued operations: Net sales ...Earnings from operations of Physio-Control ...Physio-Control divestiture-related costs ...Gain on sale of Physio Control ...Income tax expense ...Earnings from...
-
Page 90
...quarter of ï¬scal year 2012, the Company recognized a pre-tax gain on sale of $218 million, which includes a reversal of the portion of the Company's currency translation adjustment related to Physio-Control. Additionally, during ï¬scal year 2012, the Company recorded $42 million of Physio-Control...
-
Page 91
Medtronic, Inc. Notes to Consolidated Financial Statements (Continued) Fiscal Year 2011 Initiative In the fourth quarter of ï¬scal year 2011, the Company recorded a $272 million restructuring charge (including $2 million of restructuring charges related to the Physio-Control business presented as ...
-
Page 92
... date each company was acquired. Fiscal Year 2012 Salient Surgical Technologies, Inc. On August 31, 2011, the Company acquired Salient Surgical Technologies, Inc. (Salient). Salient develops and markets devices for haemostatic sealing of soft tissue and bone incorporating advanced energy technology...
-
Page 93
.... The IPR&D primarily relates to the future launch of Salient's concentric wire product. Acquired goodwill is not deductible for tax purposes. The Company accounted for the acquisition of Salient as a business combination. During ï¬scal year 2012, the Company recorded minor adjustments to other...
-
Page 94
...Control divestiture-related costs previously recorded in acquisition-related items within continuing operations on the consolidated statements of earnings in the ï¬rst and second quarters of ï¬scal year 2012 to discontinued operations. Fiscal Year 2011 Ardian, Inc. On January 13, 2011, the Company...
-
Page 95
... 12, 2010, the Company acquired ATS Medical, Inc. (ATS Medical). ATS Medical is a leading developer, manufacturer, and marketer of products and services focused on cardiac surgery, including heart valves and surgical cryoablation technology. Under the terms of the agreement, ATS Medical shareholders...
-
Page 96
... costs which were recorded within acquisition-related items in the consolidated statements of earnings. Fiscal Year 2010 In April 2010, the Company acquired privately-held Invatec S.p.A. (Invatec), a developer of innovative medical technologies for the interventional treatment of cardiovascular...
-
Page 97
... ï¬scal year 2010. In February 2010, the Company recorded an IPR&D charge of $11 million related to the asset acquisition of Arbor Surgical Technologies, Inc.'s bovine pericardial heart valve technology. These amounts were recorded within acquisition-related items in the consolidated statements of...
-
Page 98
... of contingent milestone payments associated with acquisitions subsequent to April 24, 2009 measured at fair value that used signiï¬cant unobservable inputs (Level 3):
(in millions) _____ Fiscal Year 2012 2011 _____ _____
Beginning Balance ...Purchase price contingent consideration ...Contingent...
-
Page 99
..., Inc. Notes to Consolidated Financial Statements (Continued) 6. Investments The Company invests in short-term and long-term investments, which consist primarily of marketable debt and equity securities. The carrying amounts of cash and cash equivalents approximate fair value due to their short...
-
Page 100
... to sell, before recovery of the amortized cost. Activity related to the Company's short-term and long-term investment portfolio is as follows:
Fiscal Year _____ 2012 2011 2010 Debt (a) Equity (b)(c) Debt (a) Equity (b)(d) Debt (a) Equity (b
(in millions) _____
Proceeds from sales ...Gross...
-
Page 101
..., 2012 and April 29, 2011, the aggregate carrying amount of equity and other securities without a quoted market price and accounted for using the cost or equity method was $508 million and $652 million, respectively. The total carrying value of these investments is reviewed quarterly for changes in...
-
Page 102
... Consolidated Financial Statements (Continued) 7. Fair Value Measurements The Company adopted ASC Update No. 2011-04, Fair Value Measurement (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs, in the fourth quarter of ï¬scal year 2012...
-
Page 103
.... The Level 2 derivative instruments are primarily valued using standard calculations and models that use readily observable market data as their basis. Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash ï¬,ow methodologies, or similar...
-
Page 104
... The fair value of the Company's cost or equity method investments is not estimated if there are no identiï¬ed events or changes in circumstance that may have a signiï¬cant adverse effect on the fair value of these investments. During ï¬scal years 2012, 2011, and 2010, the Company determined that...
-
Page 105
...charges in ï¬scal years 2012, 2011, and 2010, respectively. The impairment charges related to the cost method investments were recorded in other expense, net in the consolidated statements of earnings. These investments fall within Level 3 of the fair value hierarchy, due to the use of signiï¬cant...
-
Page 106
... for ï¬scal years 2012 and 2011 are as follows:
(in millions) _____ Cardiac and Restorative Vascular Group _____ Therapies Group _____ Total _____
Balance as of April 30, 2010 ...$ 1,588 $ 6,803 $ 8,391 Goodwill as a result of acquisitions ...1,028 33 1,061 Purchase accounting adjustments, net...
-
Page 107
Medtronic, Inc. Notes to Consolidated Financial Statements (Continued) The Company completed its annual intangible assets impairment review during the third quarter of ï¬scal years ended April 27, 2012, April 29, 2011, and April 30, 2010. The Company did not record any intangible asset impairments ...
-
Page 108
... or otherwise. The call options, which cost an aggregate $1.075 billion ($699 million net of tax beneï¬t), were recorded as a reduction of shareholders' equity. In separate transactions, the Company sold warrants to issue shares of the Company's common stock at an exercise price of $76.56 per...
-
Page 109
... 15 of each year, commencing September 15, 2012. The Company used the net proceeds from the sale of the 2012 Senior Notes for working capital and general corporate purposes. As of April 27, 2012 and April 29, 2011, the Company had interest rate swap agreements designated as fair value hedges of...
-
Page 110
... under credit agreements with various banks. These advances are guaranteed by the Company. Lines of Credit The Company has committed and uncommitted lines of credit with various banks. The committed lines of credit include a four-year $2.250 billion syndicated credit facility dated December 9, 2010...
-
Page 111
... are recognized currently in earnings, thereby offsetting the current earnings effect of the related change in U.S. dollar value of foreign currency denominated assets and liabilities. The cash ï¬,ows from these contracts are reported as operating activities in the consolidated statements of...
-
Page 112
... the term of the related debt. In the second quarter of ï¬scal year 2012, the Company entered into $750 million of pay ï¬xed, forward starting interest rate swaps with a weighted average ï¬xed rate of 2.84 percent in anticipation of a planned debt issuance. The market value of outstanding forward...
-
Page 113
... have been reported as operating activities in the consolidated statements of cash ï¬,ows. In March 2011, the Company entered into ï¬ve-year and ten-year ï¬xed-to-ï¬,oating interest rate swap agreements with a consolidated notional amount of $750 million, which were designated as fair value hedges...
-
Page 114
...were recorded as increases in interest expense, net on the consolidated statements of earnings. During ï¬scal years 2012, 2011, and 2010, the Company did not have any ineffective fair value hedging instruments. In addition, the Company did not recognize any gains or losses during ï¬scal years 2012...
-
Page 115
... _____
Concentrations of Credit Risk Financial instruments, which potentially subject the Company to signiï¬cant concentrations of credit risk, consist principally of interest-bearing investments, foreign exchange derivative contracts, and trade accounts receivable. The Company maintains cash and...
-
Page 116
... issuance costs and debt discounts. 12. Shareholders' Equity Repurchase of Common Stock Shares are repurchased from time to time to support the Company's stock-based compensation programs and to return capital to shareholders. In June 2009 and June 2011, the Company's Board of Directors authorized...
-
Page 117
... 2012, the last trading day before the end of the calendar quarter purchase period. At April 27, 2012, approximately 10 million shares of common stock were available for future purchase under the ESPP. Valuation Assumptions The Company uses the Black-Scholes option pricing model (Black-Scholes model...
-
Page 118
... shares recognized for ï¬scal years 2012, 2011, and 2010:
(in millions) _____ Fiscal Year 2012 2011 2010
Stock options ...Restricted stock awards ...Employee stock purchase plan ...Physio-Control award acceleration ...Total stock-based compensation expense ...Cost of products sold ...Research...
-
Page 119
Medtronic, Inc. Notes to Consolidated Financial Statements (Continued) Stock Options The following table summarizes all stock option activity, including activity from options assumed or issued as a result of acquisitions, during ï¬scal years 2012, 2011, and 2010:
Fiscal Year _____ 2012 2011 2010 ...
-
Page 120
...or credit in a tax return in future years for which the Company has already recorded the tax beneï¬t in the consolidated statements of earnings. The Company establishes valuation allowances for deferred tax assets when the amount of expected future taxable income is not likely to support the use of...
-
Page 121
... as follows:
Fiscal Year 2012 2011 2010
U.S. Federal statutory tax rate ...Increase (decrease) in tax rate resulting from: U.S. state taxes, net of Federal tax beneï¬t ...Research and development credit ...Domestic production activities ...International ...Puerto Rico Excise Tax ...Impact of...
-
Page 122
... that remain outstanding relate to the allocation of income between Medtronic, Inc. and its whollyowned subsidiary operating in Puerto Rico, which is one of the Company's key manufacturing sites, as well as the timing of the deductibility of a settlement payment. On December 23, 2010, the IRS issued...
-
Page 123
...$237 million in ï¬scal years 2012, 2011, and 2010, respectively. In the U.S., the Company maintains a qualiï¬ed pension plan designed to provide guaranteed minimum retirement beneï¬ts to all eligible U.S. employees. Pension coverage for non-U.S. employees of the Company is provided, to the extent...
-
Page 124
Medtronic, Inc. Notes to Consolidated Financial Statements (Continued) The change in beneï¬t obligation and funded status of the Company's employee retirement plans are as follows:
U.S. Pension Beneï¬ts _____ Fiscal Year _____ 2012 2011 Non-U.S. Pension Beneï¬ts _____ Fiscal Year _____ 2012 2011...
-
Page 125
... value ...
$
2,456 1,950
$ 474 225
The net periodic beneï¬t cost of the plans include the following components:
U.S. Pension Beneï¬ts ___ _____ Non-U.S. Pension Beneï¬ts _ _____ Post-Retirement Beneï¬ts _____ ___ Fiscal Year Fiscal Year Fiscal Year 2012 2011 2010 2012 2011 2010 2012 2011...
-
Page 126
... securities, growth and value styles, large cap and small cap stocks, active and passive management, and derivative-based styles. The Plan Committee believes with prudent risk tolerance and asset diversiï¬cation, the account should be able to meet its pension and other post-retirement obligations...
-
Page 127
Medtronic, Inc. Notes to Consolidated Financial Statements (Continued) The Plan did not hold any investments in the Company's common stock as of April 27, 2012 and April 29, 2011. The Company's pension plan target allocations at April 27, 2012 and April 29, 2011, by asset category, are as follows: ...
-
Page 128
... 1, 2, and 3. U.S. Pension Beneï¬ts
(in millions) _____ Fair Value at April 27, 2012 _____ Fair Value Measurements Using Inputs Considered as Level 1 Level 2 Level 3
Short-term investments ...U.S. government securities ...Corporate debt securities ...Other common stock ...Equity mutual funds...
-
Page 129
... to Consolidated Financial Statements (Continued)
Fair Value at April 29, 2011 _____ Fair Value Measurements Using Inputs Considered as Level 1 Level 2 Level 3
(in millions) _____
Short-term investments ...U.S. government securities ...Corporate debt securities ...Other common stock ...Equity...
-
Page 130
Medtronic, Inc. Notes to Consolidated Financial Statements (Continued) Post-Retirement Beneï¬ts
(in millions) _____ Fair Value at April 27, 2012 _____ Fair Value Measurements Using Inputs Considered as Level 1 Level 2 Level 3
Short-term investments ...U.S. government securities ...Corporate ...
-
Page 131
... beginning in 2013. U.S. GAAP requires the impact of a change in tax law to be recognized immediately in the income statement in the period that includes the enactment date, regardless of the effective date of the change in tax law. As a result of this change in tax law, the Company recorded a non...
-
Page 132
...scal years 2012, 2011, and 2010, respectively. 16. Leases The Company leases ofï¬ce, manufacturing, and research facilities and warehouses, as well as transportation, data processing, and other equipment under capital and operating leases. A substantial number of these leases contain options that...
-
Page 133
... of the ï¬rst lawsuit. Medtronic has moved to dismiss the lawsuit. Also pending in the Delaware court is Edwards' claim that the CoreValve transcatheter aortic valve replacement product infringes a Cribier patent. This claim is scheduled for trial in calendar year 2014. Edwards also previously...
-
Page 134
... the INFUSE bone graft product which artiï¬cially inï¬,ated Medtronic's stock price during the period. On August 21, 2009, plaintiffs ï¬led a consolidated putative class action complaint expanding the class. The Court certiï¬ed the class on December 12, 2011. On March 30, 2012, the Company...
-
Page 135
... subpoena from the Ofï¬ce of Inspector General for the Department of Health and Human Services in the Eastern District of California requesting production of documents relating to the Company's cardiac rhythm medical devices, including revenue, sales, marketing, and promotional documents, documents...
-
Page 136
...Company has not experienced signiï¬cant losses on these types of indemniï¬cations. 18. Quarterly Financial Data (unaudited)
(in millions, except per share data) _____ __ First Quarter _____ Second Quarter Third Quarter Fourth Quarter _____ Fiscal Year _____
Net Sales 2012 2011 Gross Proï¬t 2012...
-
Page 137
... end-customer revenues from the sale of products they each develop and manufacture or distribute. Net sales and earnings before income taxes by reportable segment are as follows:
(in millions) _____ _ Fiscal Year _____ 2012 __ 2011 2010
Cardiac and Vascular Group ...Restorative Therapies Group...
-
Page 138
Medtronic, Inc. Notes to Consolidated Financial Statements (Continued) Geographic Information Net sales to external customers by geography are as follows:
(in millions) _____ United States _____ Europe and Canada _____ Asia Paciï¬c _____ Other Foreign _____ ____ Consolidated _____
Fiscal Year 2012...
-
Page 139
...Registered Public Accounting Firm," which expresses an unqualiï¬ed opinion on the effectiveness of the Company's internal control over ï¬nancial reporting as of April 27, 2012, which is included in "Item 8. Financial Statements and Supplementary Data" in this Annual Report on Form 10-K. Changes in...
-
Page 140
... Shareholders," "Share Ownership Information - Beneï¬cial Ownership of Management," and "Executive Compensation - Equity Compensation Plan Information" in our Proxy Statement for our 2012 Annual Shareholders' Meeting are incorporated herein by reference. Item 13. Certain Relationships and Related...
-
Page 141
...Schedules (a) 1. Financial Statement Schedules Schedule II. Valuation and Qualifying Accounts - years ended April 27 , 2012, April 29, 2011, and April 30, 2010 (set forth on page 131 of this report). All other schedules are omitted because they are not applicable or the required information is shown...
-
Page 142
...24†10.25†10.26â€
Medtronic, Inc. Capital Accumulation Plan Deferral Program (as restated generally effective January 1, 2008)(Exhibit 10.5).(q) Stock Option Replacement Program (Exhibit 10.8).(a) Medtronic, Inc. 1998 Outside Director Stock Compensation Plan (as amended and restated effective...
-
Page 143
... Award and Incentive Plan (Exhibit 10.3).(s) Amendment No. 2 dated April 27, 2009, to Indemnification Trust Agreement (Exhibit 10.53). (v) Form of Change of Control Employment Agreement for Medtronic Executive Officers (Exhibit 10.1).(w) Medtronic, Inc. 2005 Employee Stock Purchase Plan, as amended...
-
Page 144
...to the Medtronic, Inc. Capital Accumulation Plan Deferral Program and Supplemental Executive Retirement Plan (Exhibit 10.57).(aa) Separation Agreement by and between Medtronic, Inc. and William A. Hawkins dated December 28, 2010 (Exhibit 10.1).(bb) Letter Agreement by and between Medtronic, Inc. and...
-
Page 145
... Incorporated herein by reference to the cited exhibit in our Current Report on Form 8-K, ï¬led with the Commission on March 16, 2011. (z) Incorporated herein by reference to the cited exhibit in our Annual Report on Form 10-K for the year ended April 30, 2010, ï¬led with the Commission on June 29...
-
Page 146
... reference to the cited exhibit in our Quarterly Report on Form 10-Q, for the quarter ended July 29, 2011, ï¬led with the Commission on September 7, 2011. *Exhibits that are management contracts or compensatory plans or arrangements. †Confidential treatment requested as to portions of the exhibit...
-
Page 147
... Securities Exchange Act of 1934, the report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated. MEDTRONIC, INC. Dated: June 26, 2012 By: /s/ Omar Ishrak Omar Ishrak Chairman and Chief Executive Ofï¬cer (Principal Executive Of...
-
Page 148
MEDTRONIC, INC. AND SUBSIDIARIES SCHEDULE II - VALUATION AND QUALIFYING ACCOUNTS (dollars in millions)
Balance at Beginning of Fiscal Year* _____ Charges to Earnings* _____ Other Balance Changes at End of (Debit) Credit* Fiscal Year
Allowance for doubtful accounts: Year ended 4/27/12 ...Year ...
-
Page 149
(This page has been left blank intentionally.)
132
-
Page 150
-
Page 151
...Mission
To contribute to human welfare by application of biomedical engineering in the research, design, manufacture, and sale of instruments or appliances that alleviate pain, restore health... au bien-être de l'homme en appliquant les principes de l'ingénierie biomédicale à la recherche,...
-
Page 152















