Eli Lilly 2013 Annual Report Download - page 4
Download and view the complete annual report
Please find page 4 of the 2013 Eli Lilly annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
2
To Our Shareholders
For Eli Lilly and Company, 2013 was a year of transition
and achievement. Once again, we confronted the chal-
lenges of a major patent expiration—in this instance, our
U.S. Cymbalta® patent in December. At the same time, we
completed four major regulatory lings for new products, a
record for our company.
Looking ahead, 2014 represents the most challenging
year of this period—which we’ve called “YZ”—when we lose
patent protection on several of our largest products, culmi-
nating with Evista® in March. But we have prepared for this
challenge and are positioned to return to growth and expand-
ing margins in 2015 and beyond.
Indeed, we view 2014 as a new beginning for Lilly when
we start to emerge from YZ with the anticipated launch
of three new medicines. e prospect of these launches—
with more to follow in 2015—represents the fruit of our
innovation-based strategy and is a testament to the thousands
of Lilly people who have performed so well through this
challenging period.
Since I became CEO in 2008, I’ve been candid about
both our challenges and our opportunities, as we have
rearmed Lilly’s commitment to innovation as our best path
forward to create value for patients, physicians, payers—and
for shareholders.
We’ve undertaken extensive eorts to transform our
company to address not only the challenge of patent expira-
tions, but also the demands of patients and payers alike for
greater value from medicine. We’ve delivered on our commit-
ments, we’ve adjusted to complications encountered along the
way, and we’ve positioned the company to bridge one of the
most signicant patent clis in the industry—while remain-
ing independent.
We’ve also successfully rebuilt our late-stage pipeline.
e four potential medicines we submitted this past year for
regulatory review include three to treat diabetes—dulaglutide,
empagliozin, and our new insulin glargine product—as
well as ramucirumab as a single-agent treatment in advanced
gastric cancer. In 2014, we expect to submit necitumumab
for squamous non-small cell lung cancer, as well as additional
indications for ramucirumab.
After a brief review of 2013 results, I’ll focus on the two
therapeutic areas where we expect to launch new medicines
this year—diabetes and oncology—which represent key areas
of growth for Lilly in the years ahead. And I’ll review our
broad research eorts to sustain progress in our pipeline.
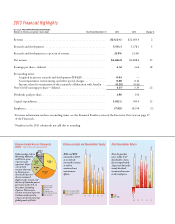
2013 Results
In 2013, revenue increased 2 percent to $23.1 billion—
following the loss of U.S. exclusivity for Cymbalta in the fourth
quarter. Even while we increased R&D spending by 5percent,
total operating expenses decreased 1percent due to lower sell-
ing and marketing expenses. Reported net income increased
15 percent, and earnings per share increased 18percent.
Eight of our products and our Elanco animal health
business exceeded $1 billion in annual sales. Japan and
China delivered double-digit volume increases, and Elanco
continued to exceed overall industry growth. is strong
performance, combined with our discipline in managing
costs, generated $5.7billion of operating cash ow, covering
capital expenditures of $1 billion and allowing the company
to return approximately $3.8billion in cash to shareholders
through the dividend and our share repurchase program.
Creating an Unmatched Portfolio of Diabetes Medicines
In 2013, Lilly took important steps to further address the
growing global epidemic of diabetes. A long-time leader in
insulins with Humulin® and Humalog®, Lilly is developing a
portfolio of diabetes medicines with unmatched breadth,
including insulins, other injectable treatments, and oral
(continued on page 4)