Medtronic 2009 Annual Report Download
Download and view the complete annual report
Please find the complete 2009 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Better Healthcare
2009 Annual Report
Table of contents
-
Page 1
Better Healthcare 2009 Annual Report -
Page 2
... year. Medtronic is headquartered in Minneapolis, Minnesota; we serve patients and physicians in 120 countries through more than 38,000 employees; and we are publicly traded on the New York Stock Exchange under the symbol MDT. Chima Miyaori Japan-Microdebrider to remove tonsils causing sleep apnea... -
Page 3
... heart valves. Aortic Disease Implantable endovascular stent grafts that provide support for a weakened and ballooning aorta, which runs through the chest and abdomen, and distributes blood from the heart to the rest of the body. Peripheral Vascular Disease* Balloon catheters and implantable stents... -
Page 4
... to control bowel function. 35 22 36 23 37 24 D i abetes 38 Diabetes Integrated diabetes management systems, insulin pumps, continuous glucose monitoring systems and therapy management software that help diabetes patients improve glucose control. †Image-Guided Surgical Systems Navigation... -
Page 5
..., restructuring, certain litigation and IPR&D charges and certain tax adjustments Dividends per share Return on equity Research and development expense Closing stock price (1) See Notes 2, 3, 4 and 13 to the consolidated financial statements for further discussion. (2) Net earnings and diluted... -
Page 6
... on us. The Foundation of Our Success Our Mission also specifically calls for us to practice "good citizenship as a company." This is in recognition that we must share our prosperity with the global communities that contribute to our success. Of course, our products and therapies carry an inherent... -
Page 7
... and diversity of our portfolio tfolio In fact, during fiscal year 2009, we held the number one portfolio. market posit ositio in five of our businesses-CRDM, Diabetes, position Neuromodulation, on, Spinal Sp S and Surgical Technologies. One Medtronic Innovation is central to our ability bility to... -
Page 8
... in continuous glucose monitoring and the development of control algorithms that are bringing the possibility of "closed loop" automated insulin delivery closer than ever before. • Surgical Technologies-we launched our Pillar technology to treat snoring and obstructive sleep apnea through... -
Page 9
Enjoying the Good Life Louis Loera (also pictured on the cover) should have been enjoying retirement in the California sunshine. But he couldn't even walk a block without tiring due to heart failure. After receiving his Medtronic cardiac resynchronization therapy-defibrillator (CRT-D), Louis ... -
Page 10
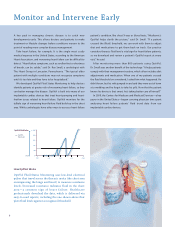
... is to catch new developments early. This allows doctors and patients to make treatment or lifestyle changes before conditions worsen to the point of needing more complex disease management. Take heart failure, for example. It is the single most costly medical expense in the United States, according... -
Page 11
...level a v vital sign for heart failure patients, so we download and d review a patient's patient' pa t's s OptiV Opti Op OptiVol iV report at every visit." Dr. Roy Small Shown with patient Donna Underhill, erhill, who has a Medtronic ICD with OptiVol er Vol The Heart Group Lancaster, Pennsylvania 7 -
Page 12
... economic value of our therapies. We can address both goals by making our therapies less invasive, so they're easier for doctors to deliver and easier on patients, helping them recover faster. One example is our endovascular stent grafts. They're used to treat abdominal and thoracic aortic aneurysms... -
Page 13
... recover from an endovascular procedure compared to open surger y. A nd t he requ i rements for anesthesia, blood products and intensive care are significantly less." Dr. Marc van Sambeek (above right) with patient Herman Kaal, who has a Medtronic Endurant Stent Graft* Catharina Hospital Eindhoven... -
Page 14
...'s glucose values in real time, offering increased visibility to blood sugar fluctuations. Phase 2: Communicating-In 2006 we introduced the first and only insulin pump integrated with CGM, which helps patients make the right insulin changes at the right time. Our CareLink Therapy Management Software... -
Page 15
... a Medtronic insulin pump integrated with continuous glucose monitoring (CGM), the nursing student was eager to make the change. "Now all I do is push two buttons to give myself insulin," he said. "No one even notices. And I have better glucose control. In the first three months of using the insulin... -
Page 16
...to our InterStim Therapy, helping us improve the lives of more patients and grow sales. InterStim treats the symptoms of voiding dysfunctions, including overactive bladder and urinary retention. It's an implantable neurostimulator that uses mild electrical pulses to continuously stimulate the sacral... -
Page 17
...much bladder and bowel* control that she had to quit her job and be housebound. Then Dr. Spinelli recommended Lina try Medtronic's InterStim Therapy. Now, she is able to serve as a foster parent and volunteers her time to help troubled teens. "Thanks to Medtronic, my life has been completely changed... -
Page 18
...could benefit from medical technologies simply aren't aware of them. So we're expanding our role to facilitate innovative marketing and distribution channels. One example is a new direct-to-consumer channel to increase awareness of our Pillar procedure for chronic snoring and obstructive sleep apnea... -
Page 19
...How the Pillar Procedure Works Most cases of snoring and obstructive sleep apnea are caused by obstructions and vibrations of the soft palate at the back of the throat. The Pillar procedure is a minimally invasive, 20-minute in-office treatment in which three tiny polyester implants are placed into... -
Page 20
... work that was 26 percent earlier than patients in the spinal fusion group. PRESTIGE was the first artificial disc approved by the U.S. Food and Drug Administration for use in the cervical spine, in July 2007. Two years later, 110 million U.S. patients have access to PRESTIGE through their insurance... -
Page 21
... of Financial Condition and Results of Operations Reports of Management Report of Independent Registered Public Accounting Firm Consolidated Statements of Earnings Consolidated Balance Sheets Consolidated Statements of Shareholders' Equity Consolidated Statements of Cash Flows Notes to Consolidated... -
Page 22
... 329 $13,515 % Change 1% 14 14 9 9 10 4 8% Cardiac Rhythm Disease Management Spinal CardioVascular Neuromodulation Diabetes Surgical Technologies Physio-Control Total Net Sales Net sales in fiscal year 2009 were $14.599 billion, an increase of 8 percent from the prior fiscal year. Foreign currency... -
Page 23
... quality system processes and outlines the actions Physio-Control must take in order to resume unrestricted distribution of our external defibrillators. We continue to work diligently to implement the required actions necessary to resolve the quality issues addressed by the FDA. Critical Accounting... -
Page 24
... Temporary differences create deferred tax assets and liabilities. Deferred tax assets generally represent items that can be used as a tax deduction or credit in our tax return in future years for which we have already recorded the tax benefit in our consolidated statements of earnings. We establish... -
Page 25
... $ 13,515 Defibrillation Systems Pacing Systems Other CARDIAC RHYTHM DISEASE MANAGEMENT Core Spinal Biologics Kyphon SPINAL Coronary Stents Other Coronary/Peripheral Endovascular Revascularization and Surgical Therapies Structural Heart Disease CARDIOVASCULAR Neuro Implantables Gastroenterology and... -
Page 26
... primarily of pacemakers, implantable defibrillators, leads, ablation products, electrophysiology catheters, products for the treatment of atrial fibrillation and information systems for the management of patients with our devices. CRDM fiscal year 2009 net sales were $5.014 billion, an increase of... -
Page 27
...therapy-pacemakers (CRT-Ps) to address the needs of patients with arrhythmias, heart failure and those at risk of sudden cardiac arrest. The Secura ICD and the Consulta CRT-D, the portfolio's first ICD and CRT-D devices, became commercially available in the U.S. in the second quarter of fiscal year... -
Page 28
..., we believe that Medtronic's growth has been slightly slower than that of the overall market. Spinal Spinal products include thoracolumbar, cervical, interbody devices, bone graft substitutes and biologic products. Spinal net sales for fiscal year 2009 were $3.400 billion, an increase of 14 percent... -
Page 29
... and peripheral stents and related delivery systems, endovascular stent graft systems, heart valve replacement technologies and tissue ablation systems, and open heart and coronary bypass grafting surgical products. CardioVascular net sales for fiscal year 2009 were $2.437 billion, an increase of 14... -
Page 30
... Abdominal Stent System expands the applicability of endovascular aortic repair to more patients with abdominal aortic aneurysms (AAA) by addressing those AAA patients whose aortas are highly angulated or whose aneurysms have short necks. Revascularization and Surgical Therapies net sales for fiscal... -
Page 31
... with our implantable pumps during the fiscal year. Movement Disorders revenue was driven by worldwide net sales of Activa Deep Brain Stimulation (DBS) Therapy. Activa DBS Therapy is used for the treatment of common movement disorders including Parkinson's disease, essential tremor and dystonia... -
Page 32
...management, our SynchroMed II drug delivery pump and our surgical lead for spinal cord stimulation, the Specify 5-6-5. Movement Disorder revenue was driven by growth in worldwide net sales of Activa DBS Therapy. Net sales of Gastroenterology and Urology products increased 10 percent over fiscal year... -
Page 33
... and sell image-guided surgery and intra-operative imaging systems. Our portfolio consists of powered tissue-removal systems and other microendoscopy instruments, implantable devices, nerve monitoring systems, disposable fluid-control products, a Ménière's disease therapy device, hydrocephalus... -
Page 34
... Stylus system. Navigation net sales for fiscal year 2008 increased 25 percent from the prior fiscal year to $159 million based on strong U.S. net sales of the O-Arm Imaging System and increased worldwide service revenue. Looking ahead, we expect our Surgical Technologies operating segment should be... -
Page 35
... in many clinical trials each fiscal year. Furthermore, we expect our development activities to help reduce patient care costs and the length of hospital stays in the future. In addition to our investment in research and development, we continue to access new technologies in areas served... -
Page 36
... development of an implantable gastric stimulator to treat obesity. Angiolink focused on the development of wound closure devices for vascular procedures. See Note 2 to the consolidated financial statements for further discussion of special charges. Restructuring Fiscal Year 2009 Initiative In the... -
Page 37
... concern Medtronic's licensed use of certain patents. The agreement reached in the fourth quarter of fiscal year 2009 ended all current and potential disputes between the two parties under their 1997 settlement and license agreement relating to coronary angioplasty stent design and balloon material... -
Page 38
...of $125 million was paid. During fiscal year 2008, we incurred certain litigation charges of $366 million. Of that amount, $123 million related to the settlement of certain lawsuits relating to the Marquis line of ICDs and CRT-Ds that were subject to a field action announced on February 10, 2005. As... -
Page 39
... tax returns and the resolution of competent authority issues for fiscal years 1992 through 2000. The $129 million certain tax benefit was recorded in the provision for income taxes in the consolidated statement of earnings for fiscal year 2007. See the "Income Taxes" section of this management... -
Page 40
... Tax Relief Act of 2008 which related to the first seven months of calendar year 2008. The remaining $28 million of operational tax benefit related to the finalization of certain tax returns, changes to uncertain tax position reserves and the impact of a state law change in 2009. During fiscal year... -
Page 41
... alternatives and may, from time to time, seek to take advantage of favorable interest rate environments or other market conditions. At April 24, 2009, our Standard and Poor's Ratings Group and Moody's Investors Service ratings remain unchanged as compared to the fiscal year ended April 25, 2008... -
Page 42
... for self-insurance coverage for our directors and officers. These investments are restricted and can only be used to indemnify or advance expenses related to claims against our directors and/or officers. We have investments in marketable debt securities that are classified and accounted for as... -
Page 43
... for the acquisition of Kyphon. Lastly, fiscal year 2009 included increased other investing activities which primarily relate to the purchase of minority investments. Although we generally invest in a number of early stage companies each year, fiscal year 2009 included the use of $221 million in... -
Page 44
... will be offset by losses/gains on the related assets, liabilities and transactions being hedged. (2) Certain leases require us to pay real estate taxes, insurance, maintenance and other operating expenses associated with the leased premises. These future costs are not included in the schedule above... -
Page 45
... repurchase common stock. As of April 24, 2009, pursuant to provisions in the indentures relating to our increase of its quarterly dividend to shareholders, the conversion rates for each of the Senior Convertible Notes is now 18.0474, which correspondingly changed the conversion price per share for... -
Page 46
... period beginning in July 2013 (the "settlement dates"). If the average price of our common stock during a defined period ending on or about the respective settlement dates exceeds the exercise price of the warrants, the warrants will be settled in shares of our common stock. Proceeds received from... -
Page 47
... CryoCath common stock, the assumption and settlement of existing CryoCath debt and the payment of direct acquisition costs. CyroCath develops cryotherapy products to treat cardiac arrhythmias. CryoCath's Arctic Front product is a minimally invasive cryo-balloon catheter designed specifically to... -
Page 48
... designed to restore and preserve spinal function using minimally invasive technology. Kyphon's primary products are used in balloon k yphoplasty for the treatment of spinal compression fractures caused by osteoporosis or cancer, and in the interspinous process decompression (IPD) procedure for... -
Page 49
... performance in Spinal, Diabetes and Surgical Technologies. Spinal net sales growth was led by growth in Core Spinal due to increased sales of the CD HORIZON family of products. Also, the acquisition of Kyphon in the third quarter of fiscal year 2008 increased the sales growth for Spinal as the... -
Page 50
..., intellectual property rights, litigation and tax matters, mergers and acquisitions, market acceptance of our products, accounting estimates, financing activities, ongoing contractual obligations and sales efforts. Such statements can be identified by the use of terminology such as "anticipate... -
Page 51
... of the Public Company Accounting Oversight Board (United States). The independent registered public accounting firm's responsibility is to express an opinion that such financial statements present fairly, in all material respects, our financial position, results of operations and cash flows... -
Page 52
Report of Independent Registered Public Accounting Firm To the Shareholders and Board of Directors of Medtronic, Inc.: In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of earnings, shareholders' equity and cash flows present fairly, in all ... -
Page 53
...(154) 8,784 3,515 713 $ 2,802 Net sales Costs and expenses: Cost of products sold Research and development expense Selling, general and administrative expense Special charges Restructuring charges Certain litigation charges Purchased in-process research and development (IPR&D) charges Other expense... -
Page 54
... Total assets Liabilities and Shareholders' Equity Current liabilities: Short-term borrowings Accounts payable Accrued compensation Accrued income taxes Other accrued expenses Total current liabilities Long-term debt Long-term accrued compensation and retirement benefits Long-term accrued income... -
Page 55
...Unrealized loss on investments Translation adjustment Net change in retirement obligations Unrealized loss on foreign exchange derivatives Total comprehensive income Dividends to shareholders Issuance of common stock under stock purchase and award plans Adjustment to deferred tax benefit recorded on... -
Page 56
...net Payments on long-term debt Issuance of long-term debt Dividends to shareholders Issuance of common stock under stock purchase and award plans Excess tax benefit from exercise of stock-based awards Repurchase of common stock Net cash used in financing activities Effect of exchange rate changes on... -
Page 57
... headquartered in Minneapolis, Minnesota, and markets its products primarily through a direct sales force in the United States (U.S.) and a combination of direct sales representatives and independent distributors in international markets. The primary markets for products are the U.S., Western Europe... -
Page 58
... of the reserve recorded is equal to the costs to repair or otherwise satisfy the claim. The Company includes the covered costs associated with field actions, if any, in warranty expense. Changes in the Company's product warranty obligations during the years ended April 24, 2009 and April 25, 2008... -
Page 59
... employees outside the U.S. Pension benefit plan costs include assumptions for the discount rate, retirement age, compensation rate increases and the expected return on plan assets. Post-retirement medical plan costs include assumptions for the discount rate, retirement age, expected return on plan... -
Page 60
... Pension and Other Postretirement Plans-an amendment of FASB Statements No. 87, 88, 106 and 132(R)" (SFAS No. 158). The tax benefit related to SFAS No. 158 was $109 million, $17 million and $92 million in fiscal years 2009, 2008 and 2007, respectively. The Company adopted the new measurement date... -
Page 61
... of common stock include stock options and other stock-based awards granted under stock-based compensation plans and shares committed to be purchased under the employee stock purchase plan. The table below sets forth the computation of basic and diluted earnings per share: Fiscal Year (in millions... -
Page 62
...in principle is recognized in the opening balance in retained earnings in the fiscal year of adoption. All other provisions of SFAS No. 157 will be applied prospectively. On February 12, 2008, the FASB issued FASB Staff Position (FSP) FAS 157-2, "Effective Date of FASB Statement No. 157" (FSP FAS No... -
Page 63
... a material impact to diluted earnings per share for the fiscal year ended April 24, 2009. In November 2008, the FASB ratified EITF Issue No. 08-6, "Equity Method Investment Accounting Considerations" (EITF No. 08-6). EITF No. 08-6 applies to all investments accounted for under the equity method and... -
Page 64
... recorded a special charge of $78 million related to the impairment of intangible assets associated with its benign prostatic hyperplasia, or enlarged prostate, product line purchased in fiscal year 2002. The development of the market, relative to the Company's original assumptions, has changed as... -
Page 65
... has entered into a five-year corporate integrity agreement effective upon dismissal of the two suits that further strengthens its employee training and compliance systems surrounding sales and marketing practices. The settlement agreement also reflects Medtronic's assertion that the Company and... -
Page 66
... in the Company's corporate functions. The asset write-downs were recorded within cost of products sold in the consolidated statement of earnings. The employee termination costs of $27 million consist of severance and the associated costs of continued medical benefits and outplacement services. As... -
Page 67
... defined benefit pension and post-retirement related expense for those employees who accepted early retirement packages. These costs are not included in the table summarizing restructuring costs below because they are associated with costs that are accounted for under the pension and postretirement... -
Page 68
... payment, including direct acquisition costs, of $700 million plus potential additional payments contingent upon achievement of certain clinical and revenue milestones. CoreValve develops percutaneous, catheter-based transfemoral aortic valve replacement products that are approved in certain markets... -
Page 69
... CryoCath common stock, the assumption and settlement of existing CryoCath debt and payment of direct acquisition costs. CryoCath develops cryotherapy products to treat cardiac arrhythmias. CryoCath's Arctic Front product is a minimally invasive cryo-balloon catheter designed specifically to treat... -
Page 70
...designed to restore and preserve spinal function using minimally invasive technology. Kyphon's primary products are used in balloon kyphoplasty for the treatment of spinal compression fractures caused by osteoporosis or cancer, and in the interspinous process decompression procedure for treating the... -
Page 71
...: the benefit of adding existing products of the Company to the portfolio of products already sold by Kyphon sales representatives; the value of Kyphon's highly trained assembled workforce; and the expected revenue growth that is attributable to expanded indications and increased market penetration... -
Page 72
... information is presented for informational purposes only. Fiscal Year (in millions, except per share data) held company. Prior to the acquisition, the Company had the exclusive rights to distribute and market the O-Arm. The O-Arm provides multi-dimensional surgical imaging for use in spinal and... -
Page 73
... balance. In connection with the acquisition of Odin, the Company acquired $9 million of technology-based intangible assets that had an estimated useful life of 12 years at the time of acquisition. Total goodwill was $12 million and was deductible for tax purposes. The results of operations related... -
Page 74
... amount of equity and other securities without a quoted market price and accounted for using the cost or equity method was $515 million and $231 million, respectively. The total carrying value of these investments is reviewed quarterly for changes in circumstance or the occurrence of events that... -
Page 75
... contracts and net investment hedges. These items were previously and will continue to be marked-to-market at each reporting period; however, the definition of fair value is now applied using SFAS No. 157. The information in the following paragraphs and tables primarily addresses matters relative to... -
Page 76
... as a result of acquisitions Purchase accounting adjustments, net Currency adjustment, net Ending balance The Company completed its impairment test of all goodwill for fiscal years ended April 24, 2009, April 25, 2008 and April 27, 2007 and concluded there were no impairments. 72 Medtronic, Inc. -
Page 77
... Five-year new senior notes Ten-year senior notes Ten-year new senior notes Thirty-year new senior notes Interest rate swaps Gain from interest rate swap termination Capital lease obligations Total Long-Term Debt $ 522 2011-2022 2011 2011 2011 2013 2014 2016 2019 2039 2011/2016 N/A 2010-2014 $ 15... -
Page 78
... common stock. As of April 24, 2009, pursuant to provisions in the indentures relating to the Company's increase of its quarterly dividend to shareholders, the conversion rates for each of the Senior Convertible Notes is now 18.0474, which correspondingly changed the conversion price per share for... -
Page 79
... at an initial conversion price of $61.81 per share; however, the Debentures are not convertible before their final maturity unless the closing price of our common stock reaches 110 percent of the conversion price for 20 trading days during a consecutive 30 trading day period. Upon conversion of... -
Page 80
... quarter of fiscal year 2009, the Company adopted SFAS No. 161, "Disclosures about Derivative Instruments and Hedging Activities." The Company uses operational and economic hedges, as well as forward exchange derivative contracts to manage the impact of foreign exchange rate changes on earnings... -
Page 81
... 36-month period. Net Investment Hedges Net investment hedges are used to hedge the long-term investment (equity) in foreign operations. For hedges that meet effectiveness requirements, the net gains/(losses) related to changes in the current rates, or spot rates, are recorded as a cumulative... -
Page 82
...the interest rate swap agreements increased the outstanding balance of the Senior Notes and is being amortized as a reduction of interest expense over the remaining life of the Senior Notes. The cash flows from the termination of these interest swap agreements are reported as operating activities in... -
Page 83
... from the common stock until 15 days after the public announcement that a person or group (the Acquiring Person) has 2007 $(382) 228 $(154) 10. Interest Expense/(Income), net Interest income and interest expense for fiscal years 2009, 2008 and 2007 are as follows: Fiscal Year (in millions) 2009... -
Page 84
...life and a four-year ratable vesting term. In fiscal year 2009, the Company granted stock options under the Medtronic, Inc. 2008 Stock Award and Incentive Plan (2008 Plan), the Medtronic, Inc. 2003 Long-Term Incentive Plan (2003 Plan) and the Medtronic, Inc. 1998 Outside Directors Stock Compensation... -
Page 85
... closing stock price on the date of grant. The following table provides the weighted average fair value of options granted to employees and the related assumptions used in the Black-Scholes model: Fiscal Year 2009 Weighted average fair value of options granted Assumptions used: Expected life (years... -
Page 86
...issues new shares when stock option awards are exercised. Cash received from the exercise of stock options for the fiscal year ended April 24, 2009 was $305 million. The Company's tax deductions related to the exercise of stock options for fiscal year 2009 were $33 million. Unrecognized compensation... -
Page 87
... Stock-based compensation Accrued liabilities Federal and state benefit on uncertain tax positions Accrued legal reserves Net operating loss and credit carryforwards Pension and post-retirement benefits Unrealized currency loss Allowance for doubtful accounts Unrealized loss on equity investments... -
Page 88
... with the settlement reached with the IRS involving the review of the Company's fiscal year 2003 and fiscal year 2004 domestic income tax returns, and the resolution of competent authority issues for fiscal year 1992 through fiscal year 2000. The $129 million certain tax benefit was recorded... -
Page 89
... and an increase to long-term accrued compensation and retirement benefits of $8 million. In the U.S., the Company maintains a qualified pension plan designed to provide guaranteed minimum retirement benefits to all eligible U.S. employees. Pension coverage for non-U.S. employees of the Company... -
Page 90
.... 158 measurement date provisions Service cost Interest cost Employee contributions Plan amendments Actuarial gain Benefits paid Medicare Part D reimbursements Special termination benefits Foreign currency exchange rate changes Projected benefit obligation at end of year Change in plan assets: Fair... -
Page 91
... benefit cost Special termination benefits Total cost for period The changes in the components of unrecognized benefit plan costs for fiscal year 2009 are as follows: (in millions) U.S. Pension Benefits $250 1 1 (6) - $246 Non-U.S. Pension Benefits $ 60 1 (1) - (13) $ 47 Post-Retirement Benefits... -
Page 92
... U.S. Pension Benefits Fiscal Year 2009 Weighted average assumptions-projected benefit obligation: Discount rate Rate of compensation increase Healthcare cost trend rate Weighted average assumptions-net periodic benefit cost: Discount rate Expected return on plan assets Rate of compensation increase... -
Page 93
... 15 16 17 105 $179 (in millions) Post-Retirement Benefits Gross Payments $ 8 9 10 11 13 96 Gross Medicare Part D Receipts $ 1 1 1 1 1 13 $18 Fiscal Year 2010 2011 2012 2013 2014 2015-2019 Total $147 Non-U.S. Plans Pension Benefits Allocation 2009 Asset Category Equity securities Debt securities... -
Page 94
... implemented two new plans including an additional defined benefit pension plan and a new defined contribution pension plan, respectively: the Personal Pension Account (PPA) and the Personal Investment Account (PIA). Employees in the U.S. hired on or after May 1, 2005 have the option to participate... -
Page 95
...court, a jury determined that the ACS Lau stent patents were valid and that Medtronic's Driver, GFX, MicroStent, S540, S660, S670, Bestent2 and S7 stents (the bare metal stents) infringe those patents. Medtronic Vascular made numerous post-trial motions challenging the jury's verdict of infringement... -
Page 96
... in opposition proceedings in the European patent office. Abbott has filed similar lawsuits against Medtronic's large vessel bare metal stents in France, Germany and Japan. In the German proceeding, a trial date is set for August 20, 2009. In France, a trial is scheduled for November 30, 2009. In... -
Page 97
... class action relating to the same subject matter. Medtronic removed the action to federal court in the District of Minnesota and filed a motion to dismiss, which is pending. In addition, class action product liability suits pending in Canada are consolidated in the Ontario Superior Court of Justice... -
Page 98
... were made as to the market acceptance and use of the Fidelis defibrillator leads to artificially inflate Medtronic's stock price. Pursuant to court order, the caption of the case was changed to Medtronic, Inc., Securities Litigation, and a consolidated putative class action complaint was filed on... -
Page 99
... violations of the Employee Retirement Income Security Act. The complaint was filed purportedly on behalf of a putative class comprised of participants and beneficiaries of the Medtronic Savings and Investment Plan whose individual accounts held shares of company stock at any time from June 28... -
Page 100
... shared infrastructures. Net sales by operating segment are as follows: Fiscal Year (in millions) 18. Segment and Geographic Information The Company functions in seven operating segments, consisting of CRDM, Spinal, CardioVascular, Neuromodulation, Diabetes, Surgical Technologies and Physio-Control... -
Page 101
...of Physio-Control products manufactured at its facility in Redmond, Washington in order to address quality system issues. The Company continues to work with the FDA to address the quality system issues that must be resolved in order to resume unrestricted distribution of its external defibrillators... -
Page 102
... taxes Provision for income taxes Net earnings Per Share of Common Stock: Basic earnings Diluted earnings Cash dividends declared Financial Position at Fiscal Year-end: Working capital Current ratio Total assets Long-term debt Shareholders' equity Additional Information: Full-time employees at year... -
Page 103
... and insider trading. The information above may also be obtained upon request from the Medtronic Investor Relations Department, 710 Medtronic Parkway, Minneapolis, Minnesota 55432, USA. Stock Exchange Listing New York Stock Exchange (symbol: MDT) Price Range of Medtronic Stock Fiscal Qtr. 2009... -
Page 104
... Neve Senior Vice President and President, Spinal and Biologics James P. Mackin Senior Vice President and President, Cardiac Rhythm Disease Management Kendall J. Powell Chairman and Chief Executive Officer, General Mills Director since 2007 Quality and Technology Committee Shirley Ann Jackson, Ph... -
Page 105
Our Mission MISSION • To contribute to human welfare by application of biomedical engineering in the research, design, manufacture, and sale of instruments or appliances that alleviate pain, restore health, and extend life. • To direct our growth in the areas of biomedical engineering where we ... -
Page 106
World Headquarters Medtronic, Inc. 710 Medtronic Parkway Minneapolis, MN 55432-5604 USA Tel: 763.514.4000 Fax: 763.514.4879 Europe, Canada and the Emerging Markets Medtronic International Trading SÃ rl Route du Molliau 31 Case Postale CH-1131 Tolochenaz Switzerland Tel: 41.21.802.7000 Fax: 41.21.802...